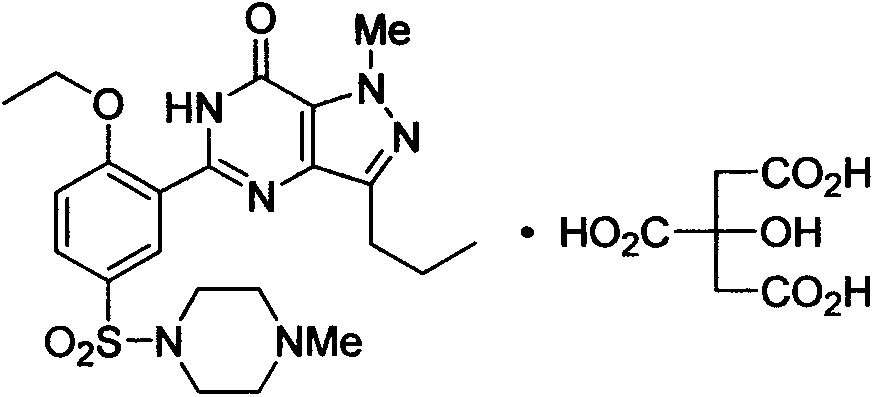
Medicinal composition containing sildenafil citrate and preparation method thereof
A technology of sildenafil citrate and sildenafil is applied in the field of pharmaceutical preparations and can solve problems such as poor taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
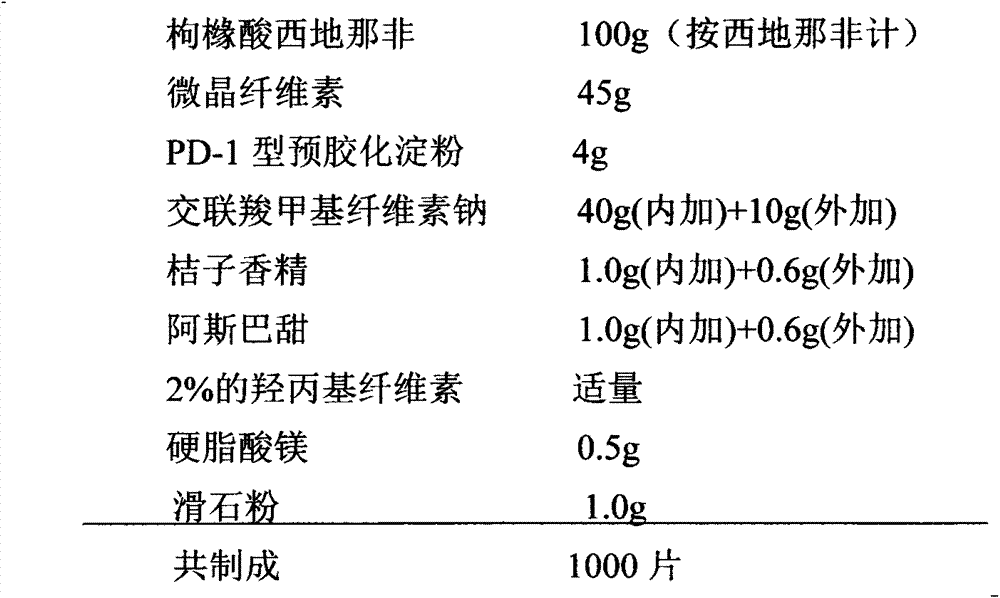
[0022] Preparation prescription of the present invention is made up of following components by tablet core weight:
[0023]
[0024] Preparation Process:
[0025] (a) Sildenafil citrate, microcrystalline cellulose, croscarmellose sodium added, orange essence and aspartame added inside are mixed through a 100-mesh sieve and added to the fluidized bed In the granulator, turn on the fan and heat to make the auxiliary materials in a fluidized state, spray the atomized 2% hydroxypropyl cellulose binder containing the added orange essence and aspartame into the granulation, and then Dry at 50-60°C, granulate with 20 meshes;
[0026] (b) Add PD-1 type pregelatinized starch, croscarmellose sodium and lubricant to the granules prepared in step (a), mix evenly, and press into tablets.
[0027] The orally rapidly disintegrating tablet containing sildenafil citrate and PD-1 type pregelatinized starch prepared in this example can completely disintegrate in 20 seconds in 37°C water, an...
Embodiment 2
[0029] Preparation prescription of the present invention is made up of following components by tablet core weight:
[0030]
[0031] Preparation Process:
[0032] (a) Sildenafil citrate, calcium hydrogen phosphate, cross-linked polyvinylpyrrolidone added, lemon essence and mannitol added inside are mixed through a 100-mesh sieve, added to a fluidized bed granulator, and Air blower, heating, so that the auxiliary materials are in a fluidized state, spray into the inside with an atomized 5% PVPK30 binder containing lemon essence and mannitol to granulate, then dry at 50-60°C, and granulate with 40 mesh .
[0033] (b) Add PD-1 pregelatinized starch, cross-linked polyvinylpyrrolidone and lubricant to the granules prepared in step (a), mix evenly, and press into tablets.
[0034] The orally rapidly disintegrating tablet containing sildenafil citrate and PD-1 type pregelatinized starch prepared in this example can completely disintegrate in 20 seconds in 37°C water, and can pas...
Embodiment 3
[0036] Preparation prescription of the present invention is made up of following components by tablet core weight:
[0037]
[0038] Preparation Process:
[0039] (a) Sildenafil citrate, lactose, croscarmellose calcium added, stevioside and aspartame added are mixed through a 120-mesh sieve and added to a fluidized bed granulator In the process, turn on the fan and heat to make the auxiliary materials in a fluidized state, spray 2% hydroxypropyl methylcellulose binder containing extra stevioside and aspartame into the inside to granulate, and then Dry at 50-60°C, granulate with 30 mesh.
[0040] (b) Add PD-1 pregelatinized starch, croscarmellose calcium and lubricant to the granules prepared in step (a), mix evenly, and press into tablets.
[0041] The orally rapidly disintegrating tablet containing sildenafil citrate and PD-1 type pregelatinized starch prepared in this example can completely disintegrate in 18 seconds in 37°C water, and can pass through a 600-710 μm sie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com