Non-aqueous electrolyte, and non-aqueous electrolyte cell using the same
A non-aqueous electrolyte and non-aqueous solvent technology, which is applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolytes, secondary batteries, etc., can solve the problem that the initial characteristics such as the charge and discharge efficiency of the first cycle have not been improved, and achieve good Film formation effect, good initial characteristics, and effect of suppressing gas generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0395] The present invention will be described in more detail with reference to synthesis examples, experimental examples, comparative experimental examples, comparative examples, and examples shown below, but the present invention is not limited to these examples.
Synthetic example
[0396] Synthesis example (methylene bissulfonate derivatives of the present invention and comparative compounds)
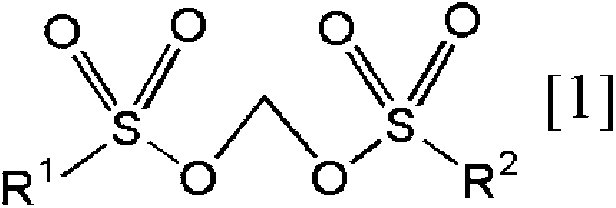
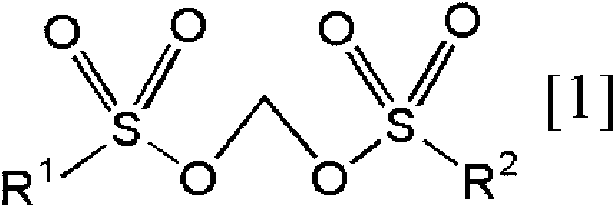
[0397] [Methylene bissulfonate of the present invention]
Synthetic example 1
[0398] Synthesis Example 1. Synthesis of Compound No.1 [Methylenebis(methanesulfonate)]
[0399]
[0400] In dimethyl carbonate (10 mL), at 55 ° C, the methylene bis (chlorosulfonate) [ClSO 2 OCH2 OSO 2 Cl] (1.5g, 6.1mmol) and pyridinium methanesulfonate (2.1g, 12.0mmol) were reacted with stirring for 3 hours. After the reaction was terminated, the precipitated pyridinium chlorosulfonate salt was filtered off, and concentrated under reduced pressure to obtain a light brown solid. After adsorption treatment with activated carbon, purification was carried out by recrystallization to obtain the target methylenebis(methanesulfonate) in a yield of 48% (0.6 g, 2.9 mmol). 1 The measurement results of H NMR are shown below.
[0401] 1 H NMR (CD 3 CN); δ=5.80(s,2H),3.19(s,6H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| D value | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com