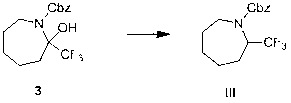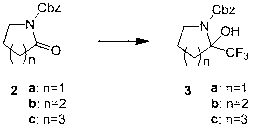Preparation method of 2-trifluoromethyl-1-carbobenzoxy-1-aza-cyclane
An azacycloalkane and benzyloxycarbonyl technology is applied in the field of preparation of 2-trifluoromethyl-1-benzyloxycarbonyl-1-azacycloalkane, and can solve the problems of low yield, unsuitability for amplification, α- Problems such as long steps to replace azacycloalkanes, to achieve the effects of low yield, improved drug-like properties, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 : 2-oxa-benzyloxycarbonyl cycloheptylamine 2c preparation
[0020]
[0021] Steps:
[0022] Under nitrogen protection, add compound cycloheptamide in a dry three-necked flask 1c (20 g, 0.23 mol) and tetrahydrofuran (200 mL), slowly added n-butyllithium (94 mL, 0.23 mol, 2.5 M n-hexane solution) under a dry-ice acetone bath. The reaction solution was stirred at -78°C for 60 minutes, and then a solution of benzyl chloroformate (40 g, 0.23 mol) in THF (50 mL) was slowly added. Slowly rise to room temperature and stir for 10 hours, quench with saturated aqueous ammonium chloride, extract with ethyl acetate, and dry and concentrate the organic phase to obtain 53 grams of product 2-oxa-benzyloxycarbonyl cycloheptylamine 2c , the yield is 100%. used directly in the next reaction.
Embodiment 2
[0023] Example 2 : 2-oxa-1-benzyloxycarbonylpyrroline 2a preparation
[0024] When n=1, the operation is the same as in Example 1. The yield is 100%. used directly in the next reaction.
Embodiment 3
[0025] Example 3 : 2-oxa-1-benzyloxycarbonylpiperidine 2b preparation
[0026] When n=2, the operation is the same as in Example 1. The yield is 70%. used directly in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com