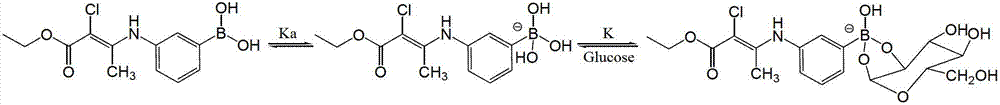
Phenylboronic acid organic gel compound
A compound, phenylboronic acid technology, applied in the field of chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
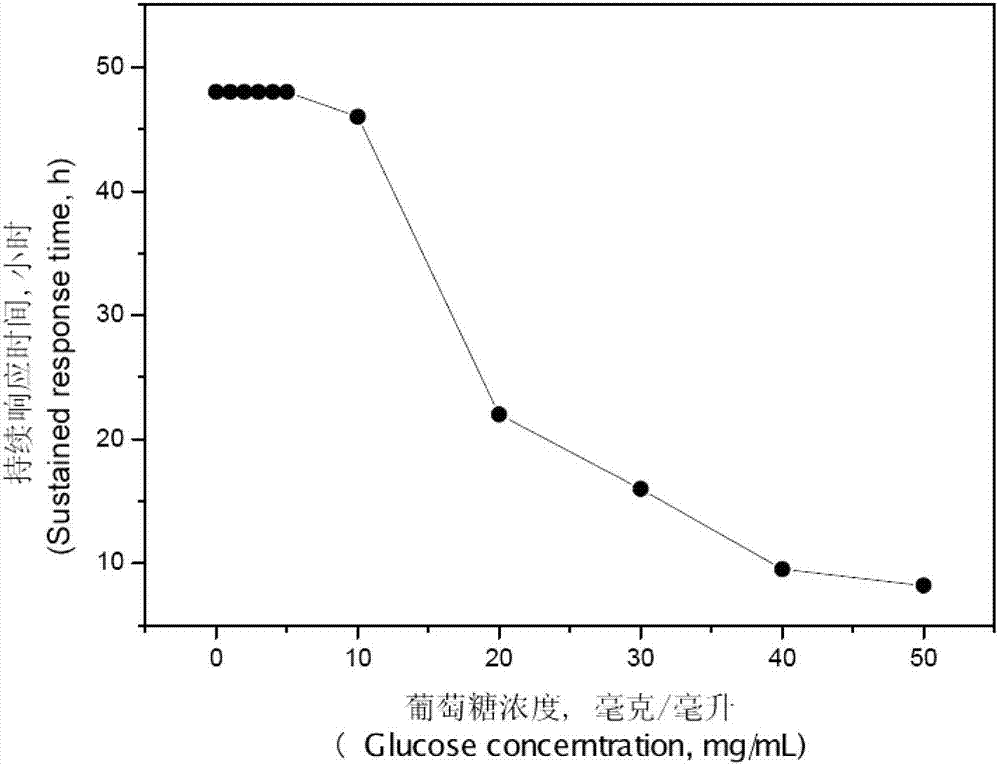
Image
Examples
Embodiment 1
[0029] Add 30 mL of chloroform, 3-aminophenylboronic acid (1.37 g, 10 mmol), and neopentyl glycol (1.04 g, 10 mmol) into a 100 mL round bottom flask, and stir at room temperature for 3 h. The reaction solution was washed 2 times with 200 mL of water, the organic phase was separated, dried with anhydrous sodium sulfate and filtered, and the organic phase was rotary evaporated to obtain compound (3) (C 11 h 16 BNO 2 , 3-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl) benzonamine), the yield was 99%. Take the compound (3) (7.0 g, 34 mmol), ethyl 2-chloroacetoacetate (6 mL, 43 mmol), add to 100 mL of dichloromethane, add triethylamine (7 mL), heat to reflux to react 24 h. After the completion of the reaction, dichloromethane was removed by rotary evaporation, extracted with ethyl acetate, and the organic phase was washed three times with saturated brine. After the organic phase was rotary evaporated, it was recrystallized with isopropanol to obtain compound (2) (C 17 h 23 BClNO 4 , e...
Embodiment 2
[0031] Add 35 mL of chloroform, 3-aminophenylboronic acid (1.37 g, 10 mmol), and neopentyl glycol (1.25 g, 12 mmol) into a 100 mL round bottom flask, and stir at room temperature for 4 h. The reaction solution was washed twice with 200 mL of water, the organic phase was separated, dried over anhydrous sodium sulfate and filtered, and the organic phase was rotary evaporated to obtain compound (3) with a yield of 99%. Take the compound (3) (7.0 g, 34 mmol), ethyl 2-chloroacetoacetate (4.7 mL, 34 mmol), add to 75 mL of dichloromethane, then add triethylamine (4.5 mL), heat to reflux React for 15 h. After the reaction was completed, dichloromethane was removed by rotary evaporation, extracted with ethyl acetate, and the organic phase was washed three times with saturated brine. After rotary evaporation, the organic phase was recrystallized with isopropanol to obtain compound (2), with a yield of 47%. Compound (2) (4.0 g, 12 mmol) was added to 40 mL of isopropanol at 5 o Diethano...
Embodiment 3
[0033] Add 40 mL of chloroform, 3-aminophenylboronic acid (1.37 g, 10 mmol), and neopentyl glycol (1.56 g, 15 mmol) into a 100 mL round bottom flask, and stir at room temperature for 2 h. The reaction solution was washed twice with 220 mL of water, the organic phase was separated, dried over anhydrous sodium sulfate and filtered, and the organic phase was rotary evaporated to obtain compound (3) with a yield of 98%. Take the compound (3) (7.0 g, 34 mmol), ethyl 2-chloroacetoacetate (62 mmol), add it to 100 mL of dichloromethane, then add triethylamine (9 mL), heat and reflux for 30 h . After the reaction was completed, dichloromethane was removed by rotary evaporation, extracted with ethyl acetate, and the organic phase was washed three times with saturated brine. After rotary evaporation, the organic phase was recrystallized with isopropanol to obtain compound (2), with a yield of 60%. Compound (2) (4.0 g, 12 mmol) was added to 60 mL of isopropanol at 0 o Diethanolamine (18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com