Slow-release composition containing L-milnacipran and preparation method thereof
A technology of levomilnacipran and sustained-release composition, which is applied in the field of sustained-release composition containing levomilnacipran and its preparation, and can solve the problems of inability to achieve consistent release behavior, poor stability, and low production efficiency, etc. problems, to achieve the effect of easy promotion, good stability, and simple production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062]
[0063] Mix levomilnacipran with calcium hydrogen phosphate and hypromellose K4M evenly, and set the mixing speed at 20-50 rpm; add 5% hypromellose aqueous solution to prepare wet granules, and granulate Stirring speed is set as: 20-50 rpm, cutting knife speed is 30-80 rpm; the above wet particles are boiled and dried, the drying temperature is set at 50-90 degrees, and the drying time is 10-30 minutes; Arrange the dried granules to obtain uniform dry granules, then add magnesium stearate and mix evenly, the mixing speed is 10-30 rpm, and the mixing time is 5-30 minutes; press the above mixture into tablets, The speed is 10-50 rpm, and the hardness is controlled at 5-13kg.
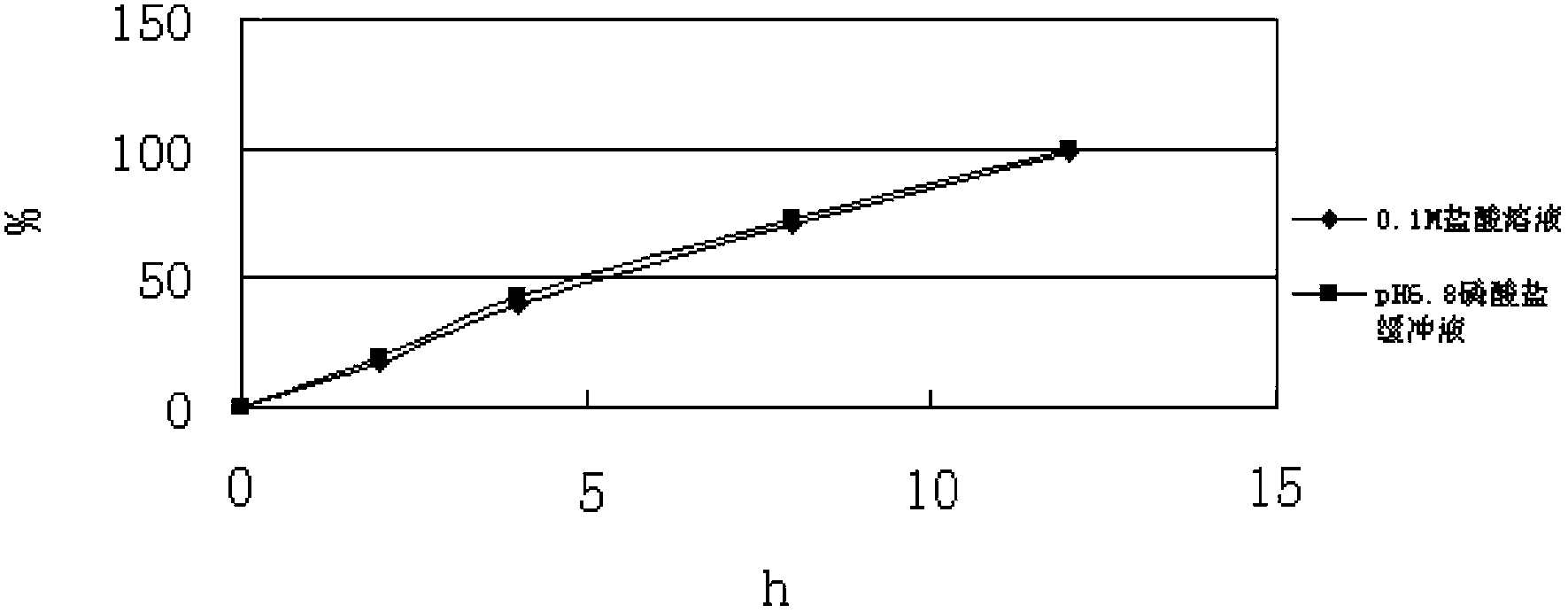
[0064] In this case, only hypromellose was used as the skeleton material, calcium hydrogen phosphate was used as the filler, and no pH regulator was used.
[0065] Take this product, according to the release test method (Chinese Pharmacopoeia 2010 edition two appendix X D first method), using t...
Embodiment 2
[0070] This embodiment is only used for comparison and description, and is not the content of the present invention.
[0071]
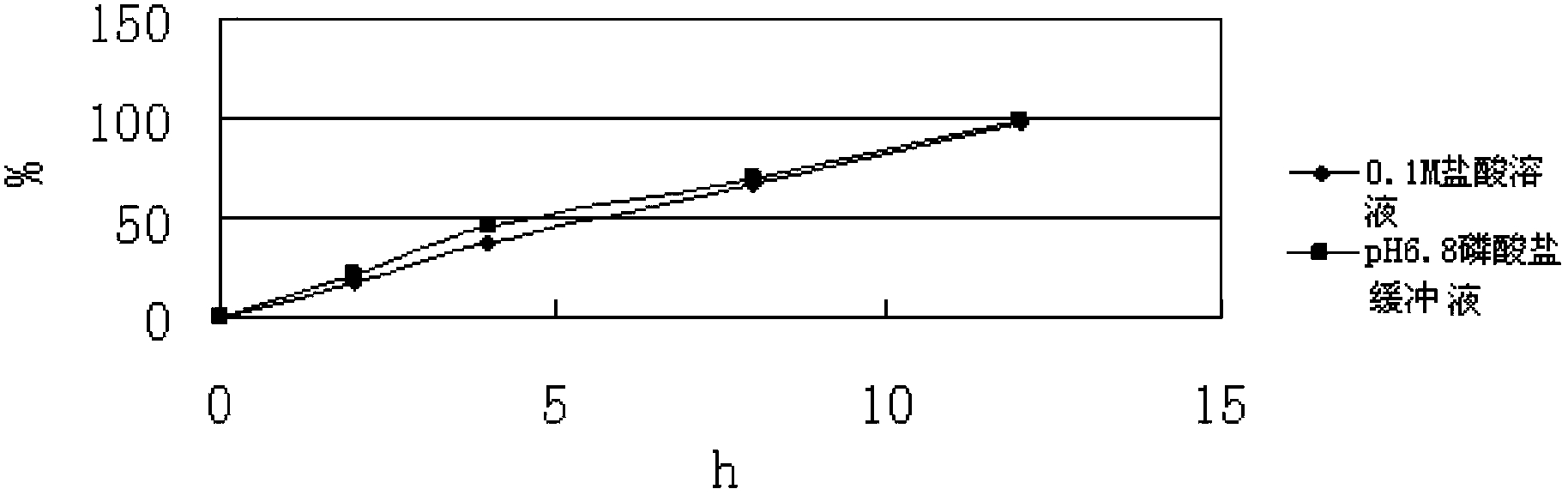
[0072] Mix levomilnacipran with calcium hydrogen phosphate and acrylic resin evenly, and set the mixing speed at 20-50 rpm; add 5% hydroxypropyl cellulose aqueous solution to prepare wet granules, and set the stirring speed for granulation to It is: 20-50 rev / min, cutting knife rotating speed is 30-80 rev / min; the above-mentioned wet granules are boiled and dried, the drying temperature is set at 50-90 degrees, and the drying time is 10-30 minutes; the dried Granules are arranged to obtain uniform dry granules, then magnesium stearate is added and mixed evenly, the mixing speed is 10-30 rpm, and the mixing time is 5-30 minutes; the above mixture is pressed into tablets, and the tableting speed is 10-30 minutes. 50 rpm, the hardness is controlled at 5-13kg.
[0073] In this case, only acrylic resin is used as the skeleton material, and calcium hydr...
Embodiment 3
[0080]
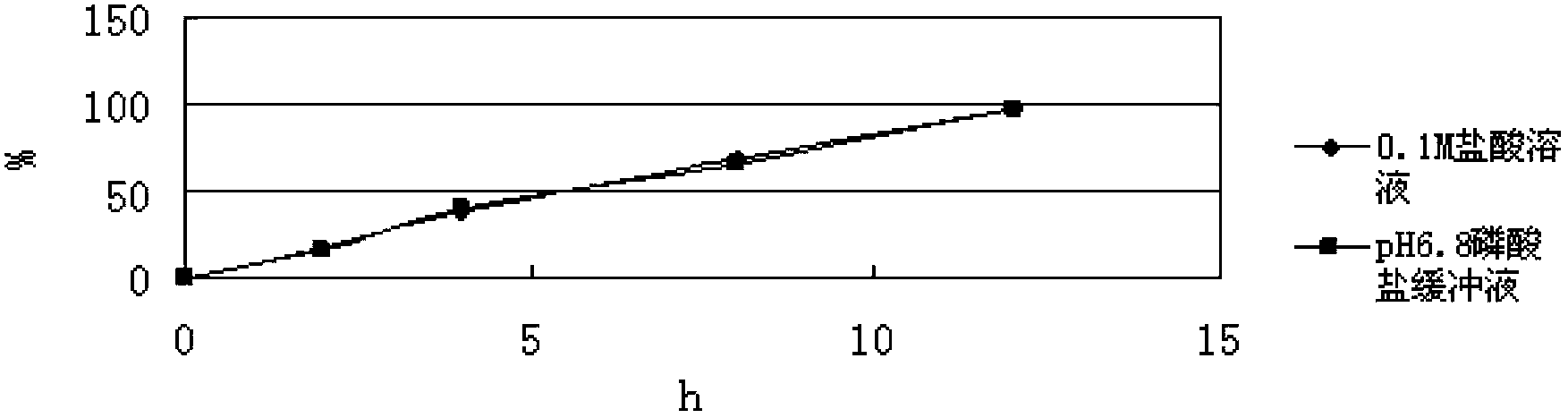
[0081] Mix levomilnacipran with calcium hydrogen phosphate and hypromellose K100M evenly, set the mixing speed at 20-50 rpm; then add acrylic resin (NE30D) and mix evenly, then add 5% hydroxyl The aqueous solution of propyl cellulose prepares wet granules, and the stirring speed of granulation is set as: 20-50 rpm, and the cutting knife rotating speed is 30-80 rpm; the above-mentioned wet granules are boiled and dried, and the drying temperature is set at 50-50 rpm. 90 degrees, the drying time is 10-30 minutes; arrange the dried granules to obtain uniform dry granules, then add magnesium stearate and mix evenly, the mixing speed is 10-30 rpm, and the mixing time is 5-30 minutes ; The above mixture is compressed into tablets, the tableting speed is 10-50 rpm, and the hardness is controlled at 5-13kg.
[0082] In this prescription, hypromellose is used as the skeleton material, calcium hydrogen phosphate is used as the filler, and acrylic resin (NE30D) is used as the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com