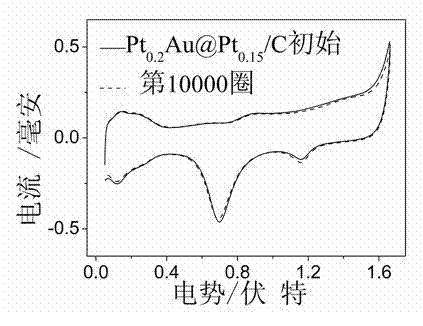
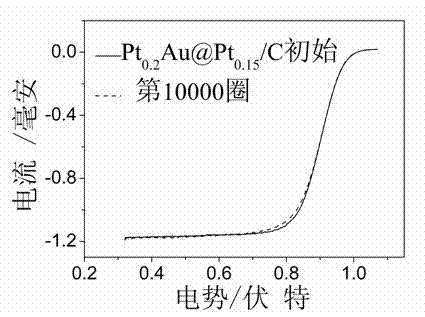
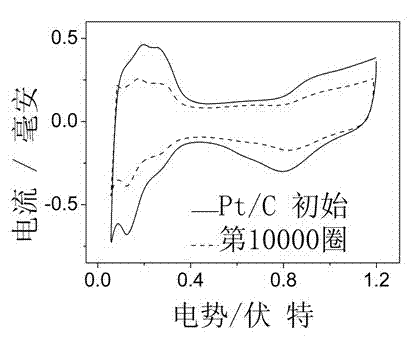
Pt-Au@Pt core-shell structure fuel cell cathode catalyst and preparation method thereof
A fuel cell cathode, core-shell structure technology, applied in battery electrodes, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of low coverage, low catalytic activity, low Pt coverage on the catalyst surface, etc. The effect of improving quality and activity, enriching resources, and solving the problem of catalyst resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method comprises steps: 1) adding sodium borohydride to the mixed solution of the gold compound and sodium citrate to reduce the gold compound to obtain Au nanoparticles. After that, the Au particles are supported on the surface of the carbon carrier to obtain carbon-supported Au, which is denoted as Au / C.
[0034] 2) Put Au / C in an aqueous solution of a platinum compound without adding a reducing agent, let Pt spontaneously reduce on the surface of Au, centrifuge and dry to obtain supported Pt-Au alloy nanoparticles, wherein the reduction temperature is 20~100 ℃, the concentration of platinum compound is 10 -5 mol / L ~10 -2 mol / L.
[0035] 3) The Pt-Au alloy nanoparticles obtained by spontaneous reduction are coated on the surface of the electrode, and the Cu atomic layer is obtained by the method of underpotential deposition, and the Cu prepared by this method is called UPD Cu. Soaking the electrode in the replacement solution allows the Pt to repl...
Embodiment 1
[0038] 1) Pt-AuPt / C core-shell structure catalyst Pt 0.2 AuPt 0.15 Preparation of / C
[0039] In the mixed solution of chloroauric acid and sodium citrate, add sodium borohydride to reduce chloroauric acid, stir evenly, add a conductive carrier after the solution turns purple, immerse at room temperature for 36 hours, centrifuge and vacuum dry to obtain carbon-loaded Au, namely Au / C;. Put Au / C at a concentration of 10 -4 mol / L ~10 -3 In the aqueous solution of the potassium chloroplatinite of mol / L, stir 24 hours at 25 degrees, obtain Pt 0.2 Au / C alloy. Pt 0.2 The Au / C alloy is coated on the surface of the electrode, the electrode potential is controlled, and Cu is deposited at a constant potential under the underpotential deposition potential of Cu to obtain an electrode with a Cu atomic layer. Soak the electrode with UPD Cu monolayer in the replacement solution potassium chloroplatinite solution for 30 min, so that Pt replaces the Cu atoms on the electrode surface, ...
Embodiment 2
[0046] 1) Pt-AuPt / C core-shell structure catalyst Pt 0.1 AuPt 0.17 Preparation of / C
[0047] In the mixed solution of chloroauric acid and sodium citrate, add sodium borohydride to reduce chloroauric acid, stir evenly, add a conductive carrier after the solution turns purple, immerse at room temperature for 48 hours, centrifuge and vacuum dry to obtain carbon-loaded Au, that is, Au / C; put Au / C at a concentration of 10 -5 mol / L ~10 -4 In the aqueous solution of potassium chloroplatinite of mol / L, stir 12 hours at 30 degree, obtain Pt 0.1 Au / C alloy. Pt 0.1 The Au / C alloy is coated on the surface of the electrode, the electrode potential is controlled, and Cu is deposited at a constant potential under the underpotential deposition potential of Cu to obtain an electrode with a Cu atomic layer. Soak the electrode with UPD Cu monolayer in the replacement solution potassium chloroplatinite solution for 15 min, so that Pt can replace the Cu atoms on the surface of the electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com