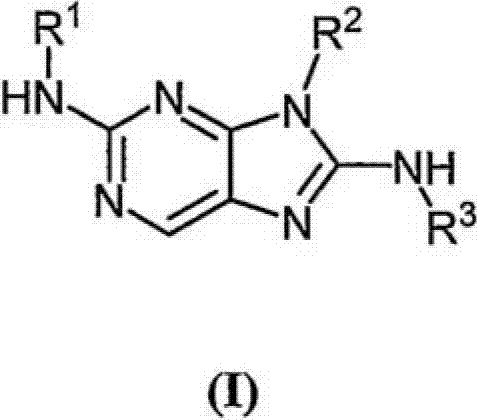
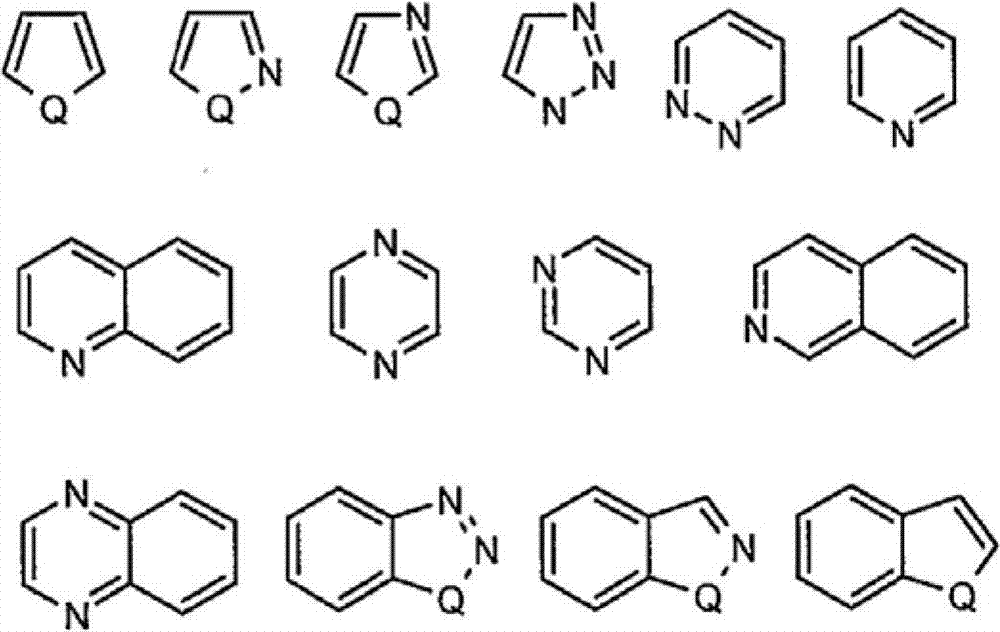
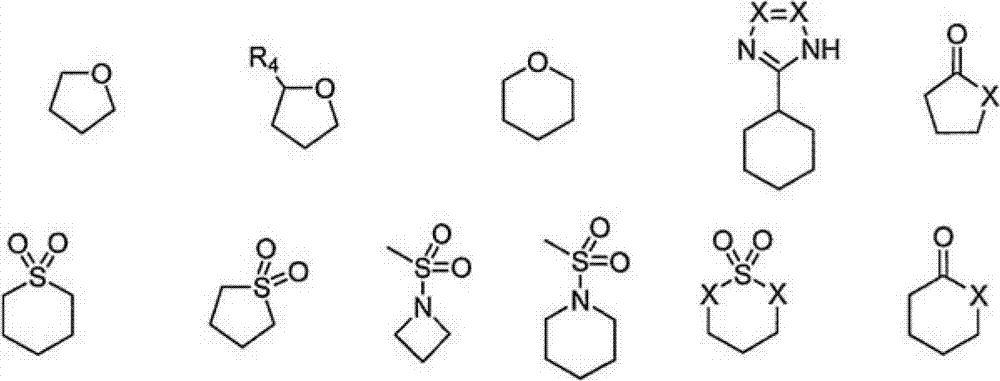
Haloaryl Substituted Aminopurines, Compositions Thereof, And Methods Of Treatment Therewith
A technology of aryl and compound, applied in haloaryl substituted aminopurine, its composition and its therapeutic field, capable of solving problems such as incomplete response to treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 51
[0357] Example 5.1: Synthesis of 4-({8-[(2,6-difluorophenyl)amino]-9-cyclopentylpurin-2-yl)amino)trans-cyclohexyl-1-alcohol
[0358]
[0359] 1. (2-Chloro-5-nitropyrimidin-4-yl)cyclopentylamine
[0360] 2,4-Dichloro-5-nitropyrimidine (10.31 mmol, 2 g) and cyclopentylamine (10.31 mmol, 1.02 mL) were dissolved in THF (60 mL), cooled to -78°C. N,N-Diisopropylethylamine (10.31 mmol, 1.8 mL) was added dropwise. The reaction mixture was stirred at -78°C for about 45 minutes. The cooling bath was removed and the reaction mixture was stirred at room temperature for about 16 hours. After removal of solvent, the residue was redissolved in EtOAc, washed with water and brine. MgSO for organic layer 4 After drying, the solvent was evaporated. The residue was subjected to column chromatography (SiO 2 , 9:1 n-hexane / ethyl acetate) to obtain the desired product (2.11g, yield 84%). ES-MS: 242 (M+l). When replacing the aforementioned cyclopentylamine with amine hydrochloride, 2-3 eq...
Embodiment 52
[0367] Example 5.2: Synthesis of trans-(4-aminocyclohexyl){8-[2,4-difluorophenyl]amino]-9-cyclopentylpurin-2-yl}amine
[0368]
[0369] 1. trans-(4-aminocyclohexyl){8-[2,4-difluorophenyl]amino]-9-cyclopentylpurin-2-yl}amine
[0370] N-[4-({8-[(2,4-difluorophenyl)amino]-9-cyclopentylpurin-2-yl}amino)trans-cyclohexyl](tert-butoxy)methyl The amide (0.71 mmol, 375 mg) was dissolved in ethanol (6 mL) and cooled to 0°C. Acetyl chloride (3 mL) was added dropwise, and the reaction was carried out at room temperature and stirred overnight. The precipitate was filtered off, washed with ether, and dried under high vacuum to afford 372 g (98% yield) of the trihydrochloride. ES-MS: 428 (M+l).
[0371] Alternatively, N-[4-({8-[(2,4-difluorophenyl)amino]-9-cyclopentylpurin-2-yl}amino)trans-cyclohexyl](tert-butyl Oxy)formamide was dissolved in 9 mL of dichloromethane, followed by the addition of 2.25 mL of TFA. The reaction mixture was stirred for about 2 hours. The solvent was remo...
Embodiment 53
[0372] Example 5.3: Synthesis of 8-(2-fluorophenylamine)-2-(4-methoxyphenylamine)-9-(trans-4-(methylamino)cyclohexyl)-9H-purine
[0373]
[0374] The Boc-protected amine (481 mg, 0.88 mmol) was dissolved in THF (6 mL) and lithium aluminum hydride (1.0 M in THF, 2.64 mL, 2.64 mmol) was added. The reaction mixture was heated to 65°C overnight. The reaction mixture was cooled to 0 °C, and the reaction was quenched by adding water dropwise until hydrogen gas no longer appeared. The precipitate was filtered off and washed well with ethyl acetate. The solvent was removed in vacuo and the residue was purified by semi-preparative HPLC (20% acetonitrile / water (0.1% TFA) → 80% acetonitrile / water (0.1% TFA), 30 min) to give 191 mg of product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com