Treatment method of copper etching waste liquid
A treatment method, copper etching technology, applied in the direction of metallurgical wastewater treatment, water/sewage treatment, chemical instruments and methods, etc., can solve the problems of large-scale, increasing the amount of treatment liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0061] Next, experimental examples and working examples are given.
[0062]
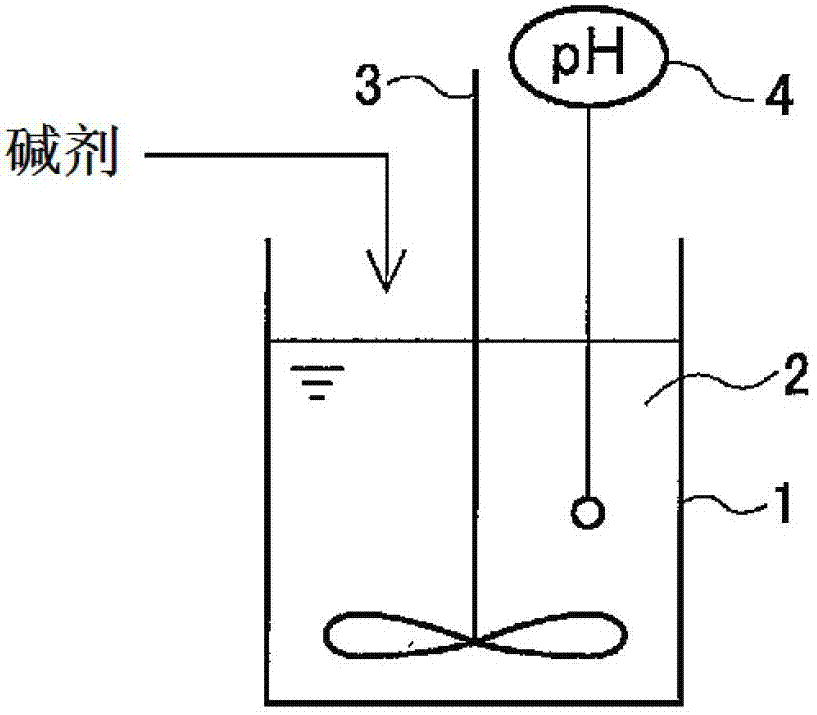
[0063] The pH of the copper etching waste solution (pH 2.2) of the following composition was not adjusted, and the pH of the copper etching waste solution (pH 2.2) was left as it was, and the change over time of the concentration of hydrogen peroxide in the copper etching waste solution was checked. As a result, the copper etching waste after one day passed The hydrogen peroxide concentration of the liquid was 2.9% by weight, and the hydrogen peroxide concentration hardly decreased. That is, it can be seen from this that hydrogen peroxide in the copper etching waste liquid is not decomposed when left as it is.
[0064] (composition of copper etching waste solution)
[0065] Hydrogen peroxide: 3% by weight
[0066] Copper: 0.4% by weight
[0067] Total nitrogen: 0.8% by weight
[0068] TOC: 1.1% by weight
[0069]
[0070] Sodium hydroxide was added to the copper etching waste liquid of the s...
Embodiment 2
[0075] For the copper etching waste liquid (pH2.2 of hydrogen peroxide concentration 6% by weight, copper concentration 0.7% by weight, all the other ingredients are identical with the copper etching waste liquid in Experimental Example 1), adjust pH in the same way as in Example 1 , and the relationship between the pH adjustment value and the hydrogen peroxide concentration of the copper etching waste liquid after 1 hour was studied. The results are shown in Table 2. In Table 2, the temperature increase (Δt) of the liquid temperature of the copper etching waste liquid after 1 hour is collectively described.
[0076] Table 2
[0077] pH(one)
[0078] As can be seen from Table 2, if the pH of the copper etching waste liquid is adjusted to more than 4, especially when it is more than 6, the hydrogen peroxide in the copper etching waste liquid can be effectively decomposed and removed in a short time of 1 hour. The decomposition of hydrogen is an exothermic reaction, ...
Embodiment 3、4
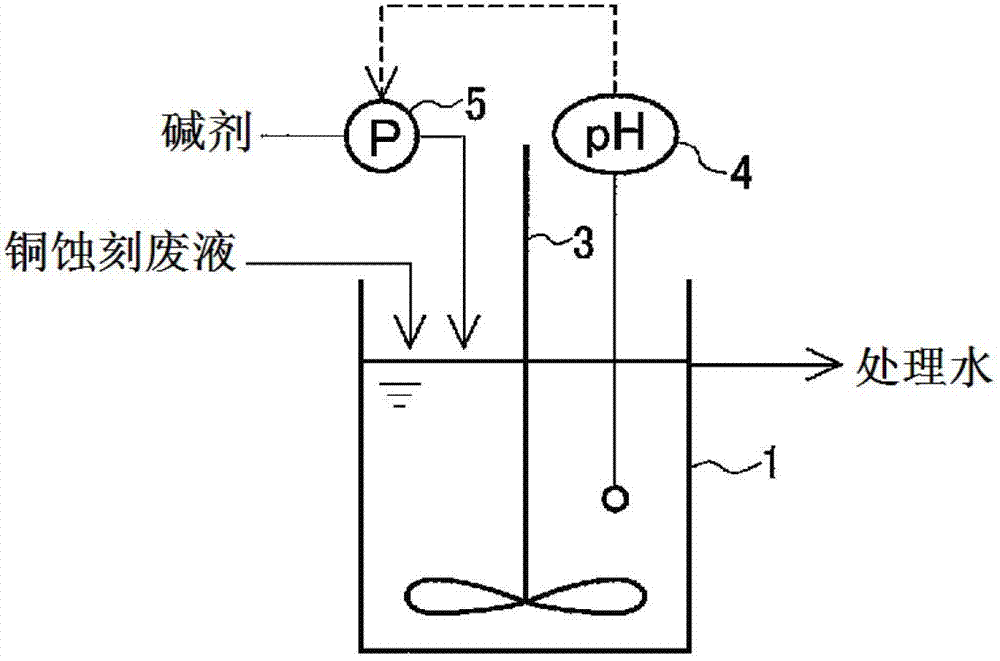
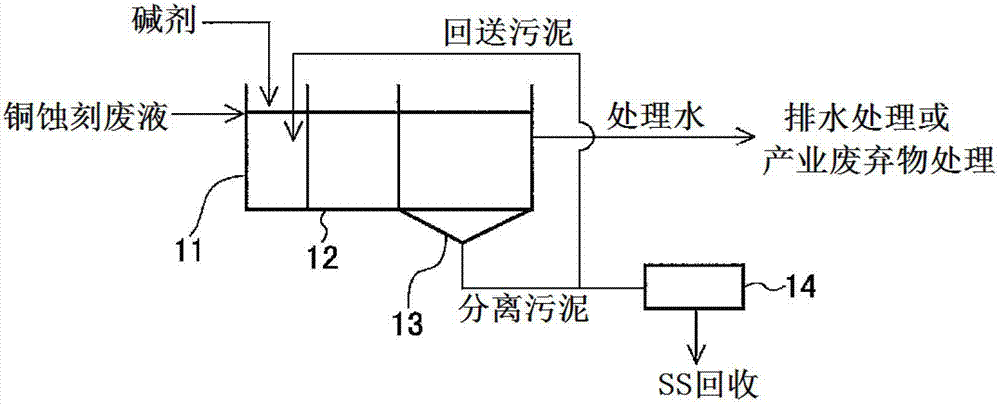
[0080] With the copper etching waste liquid of the same composition as the copper etching waste liquid processed in embodiment 2 as stock solution, adopt figure 2 In the shown reaction tank, the treatment is carried out in a continuous manner. Fill the reaction tank 1 with treated water in advance, so that the stock solution can circulate according to the specified flow rate. Sodium hydroxide was added as an alkaline agent in the reaction tank 1 to adjust the pH of the liquid in the tank to 7 (Example 3) or 8 (Example 4). The volume of the reaction tank 1 is twice the flow rate of the stock solution per hour, and the residence time of the stock solution in the reaction tank 1 is 2 hours. Table 3 shows the relationship between the liquid passing time at this time and the hydrogen peroxide concentration of the obtained treated water.
[0081] table 3
[0082]
[0083] As can be seen from Table 3, according to the present invention, even if it is a continuous treatment, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com