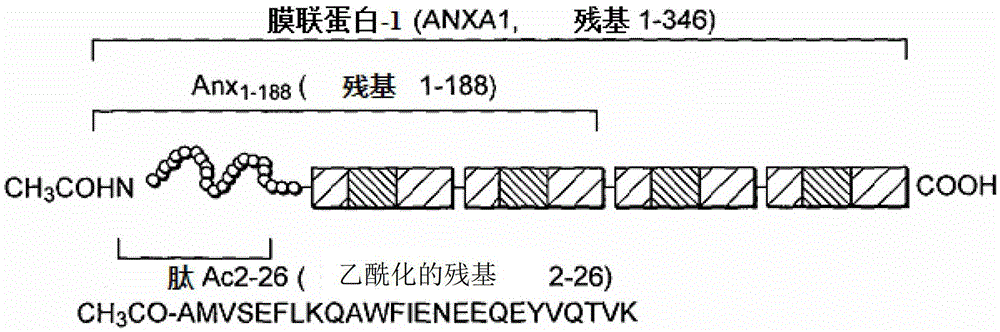
Annexin 1 antibody
An antibody, monoclonal antibody technology, applied in the direction of antibodies, immunoglobulins, antibody medical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] For the preparation of solutions and elixirs, excipients which may be used include, for example, water, polyols and sugars. For the preparation of suspensions, oils such as vegetable oils may be employed to provide oil-in-water or water-in-oil suspensions.
[0079] Pharmaceutical compositions adapted for transdermal administration may be presented in discrete patches intended to remain in intimate contact with the epidermis of the recipient over an extended period of time. For example, the active ingredient can be released from the patch by iontophoresis as generally described in Pharmaceutical Research, 3(6):318 (1986).
[0080] Pharmaceutical compositions adapted for topical administration can be formulated as ointments, creams, suspensions, lotions, powders, solutions, pastes, gels, sprays, aerosols or oils. For infections of the eye or other external tissues such as the mouth and skin, it is preferred to use the composition in the form of a topical ointment or crea...
Embodiment 1-VJ-4
[0139] Example 1 - VJ-4B6 specifically inhibits T cell activation in Annexin-1 containing T cells
[0140] Materials and methods
[0141] mice ( Figure 4B, 5, 6, 7, 8, 9, and 10)
[0142] Balb / C mice were from B&K Universal (Grimston, England). AnxA1 - / - Mice were from the inventor's laboratory, born under sterile conditions at B&K Universal. All mice used in these studies were aged 6-8 weeks. Animal experiments were carried out in accordance with the provisions of the British Home Office Regulations (Guidance on the Operation of Animals, Scientific Procedures Act 1986) and European Union regulations.
[0143] Extraction of mouse T cells ( Figure 4B , 5, 6, 7, 8, 9 and 10 cells used)
[0144] Spleens and lymph nodes (axillary, inguinal, and intestinal) were obtained from 6- to 8-week-old mice and prepared by gentle tissue degradation with a syringe plunger and a 50 μm cell strainer (BD) as previously described. The cell suspension was layered with Ficoll to obtain mo...
Embodiment 2
[0164] The sequencing of embodiment 2-VJ-4B6
[0165]The purpose of this example is to clone the genes of antibody heavy and light chain variable regions from hybridoma cells and to determine the DNA sequence and location of complementarity determining regions (CDRs) and other characteristics.
[0166] Cloning and sequencing of antibody variable regions
[0167] Total RNA was prepared from 1 vial of hybridoma cells using Qiagen RNeasy Mini Kit (CatNo:74104). RNA was eluted with 50 μL of water and checked on a 1.2% agarose gel.
[0168] V H and V K (variable kappa light chain) cDNAs were prepared using reverse transcriptase and primers for IgG and kappa constant regions. First strand cDNAs can be amplified by PCR using a large set of signal sequence primers. Amplified DNAs were purified on silica gel and cloned into vectors TEasy (Promega). Filtered V H and V K clone to confirm that it contains an insert of the appropriate size. The DNA sequence of selected clones wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com