Application of purslane alkaloid monomeric compound in preparation of anti-tumor medicament
An anti-tumor drug, alkaloid technology, applied in the field of medicine, can solve the problem that the anti-tumor activity has not been reported in literature and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Extraction, separation and structural identification of purslane amide B and orange piperonamide.
[0019] Purslane medicinal material (about 100 kg) was purchased, stirred and extracted with absolute ethanol 3 times, each time for 1 hour, and the ethanol was recovered to obtain a black extract. Add 10% sulfuric acid to the extract to dissolve and filter. The pH was adjusted to 10.0 with ammonia water, and a saturated NaCl aqueous solution was added. Dichloromethane was extracted to obtain a dichloromethane layer, which was evaporated to dry to obtain an extract rich in alkaloid extract. The extract of alkaloid extract has undergone repeated silica gel chromatography, Sephadex LH-20, ODS and liquid phase separation methods, combined with physical and chemical properties and modern spectroscopy methods (UV, IR, MS, 1H-NMR, 13C-NMR and 2D- NMR) isolated and identified two monomeric compounds, namely purslane amide B and orange piperonamide.
Embodiment 2
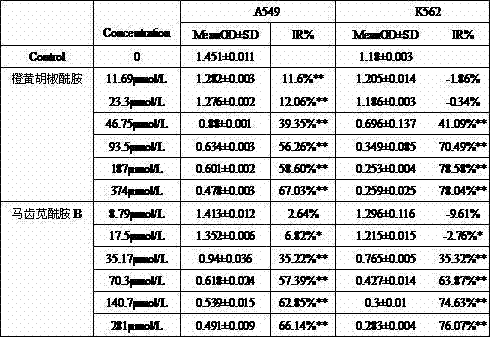
[0020] Example 2: Study on the antitumor activity of purslane amide B and orange piperonamide in vitro.
[0021] The inhibitory activities of purslane amide B and orange piperonamide on human lung adenocarcinoma cells (A549) and human leukemia cell lines (K562) were determined by MTT method.
[0022] (1) Instruments and reagents: RPMI 1640 culture medium and DMEM medium were purchased from Hyclone Company; fetal bovine serum was from Hangzhou Sijiqing; CKX31 inverted microscope (Olympus, Philippines); constant temperature CO2 incubator (Thermo, USA) ); 5810 R desktop high-speed low-temperature centrifuge (Eppendorf, Germany); automatic microplate reader (Biotek Group, USA).
[0023] (2) Cell culture: A549 cells were cultured in DMEM medium (containing 10% fetal bovine serum), K562 were cultured in 1640 medium (containing 10% fetal bovine serum), 37°C, containing 5% CO 2 cultured in a humidified incubator. Passage every 2-3 days. During the experiment, the cells in the logar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com