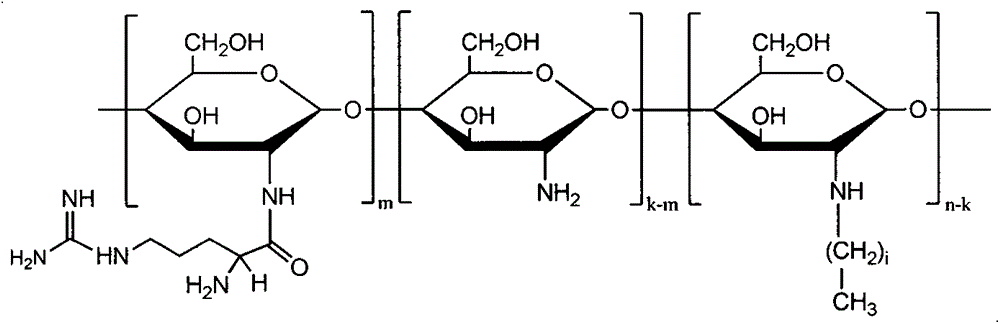
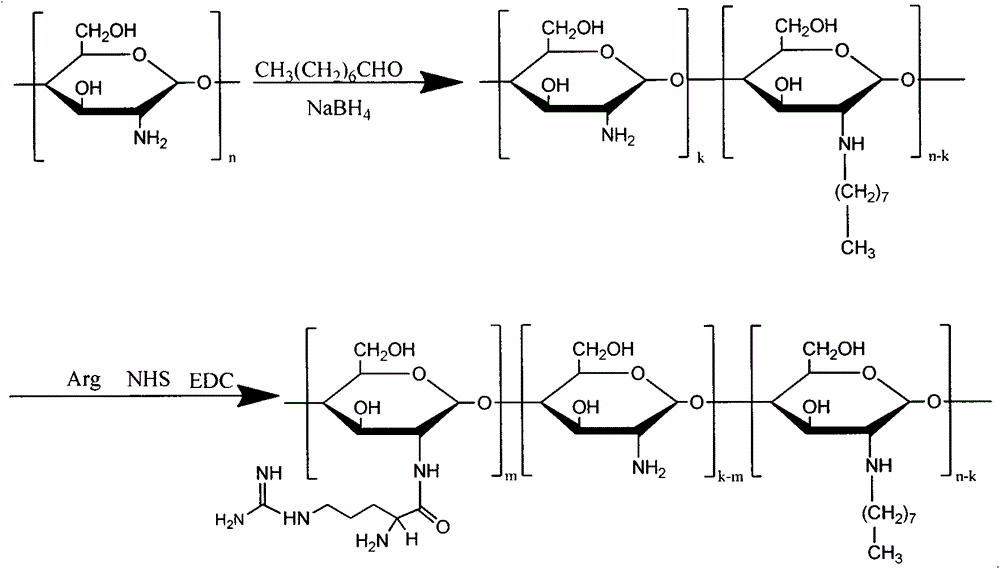
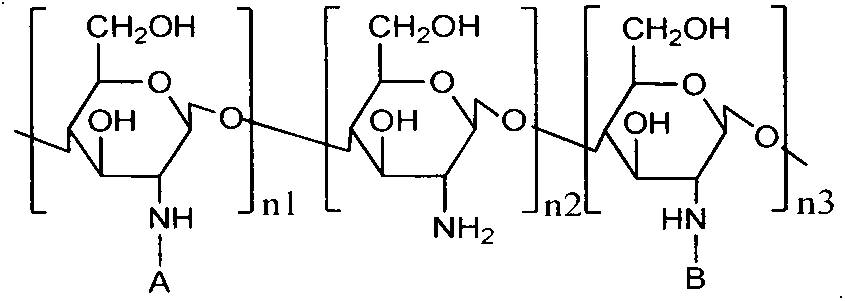
Application of using amphiphilic N-long-chain alkyl-N-arginine chitosan as solubilizing and absorption prompting carrier of gambogic acid
A technology of arginine chitosan and long-chain alkyl, which can be applied to medical preparations containing active ingredients, organic active ingredients, and pharmaceutical formulas, and can solve problems such as the unclear mechanism of arginine penetration enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Direct dissolution method:
[0054] (1) Weigh 1 gram of OACS, add 50 ml of water, and stir in a water bath at 50°C for 30 minutes to prepare a carrier solution;
[0055] (2) Add 1 gram of gambogic acid to the carrier solution, sonicate the probe under ice bath for 30 minutes, dialyzed with distilled water, and filter to obtain the drug-loaded micelle solution.
Embodiment 2
[0056] Example 2: Solvent emulsification and volatilization method: Weigh 1 gram of OACS, add 50 ml of water, stir in a water bath at 50°C for 30 minutes, weigh 1 gram of gambogic acid, and dissolve it in a dichloromethane solution to make 10g / L of gambogic acid The dichloromethane solution was added dropwise to the carrier solution, the probe was sonicated for 10 minutes in an ice bath, the dichloromethane was evaporated and the dichloromethane was evaporated overnight, and the drug-loaded micelle solution was obtained by filtering with a 0.45μm filter membrane.
Embodiment 3
[0057] Example 3: Dialysis method: Weigh 1 gram of OACS, add 50ml of water, stir for 30min in a water bath at 50°C, weigh 1 gram of GA and dissolve it in an appropriate amount of ethanol to make the concentration 10g / L, and add the ethanol solution of GA to the carrier dropwise In the solution, the probe was sonicated for 10 minutes in an ice bath, redistilled water was dialyzed overnight, and filtered with a 0.45 μm filter membrane to obtain the drug-loaded micelle solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com