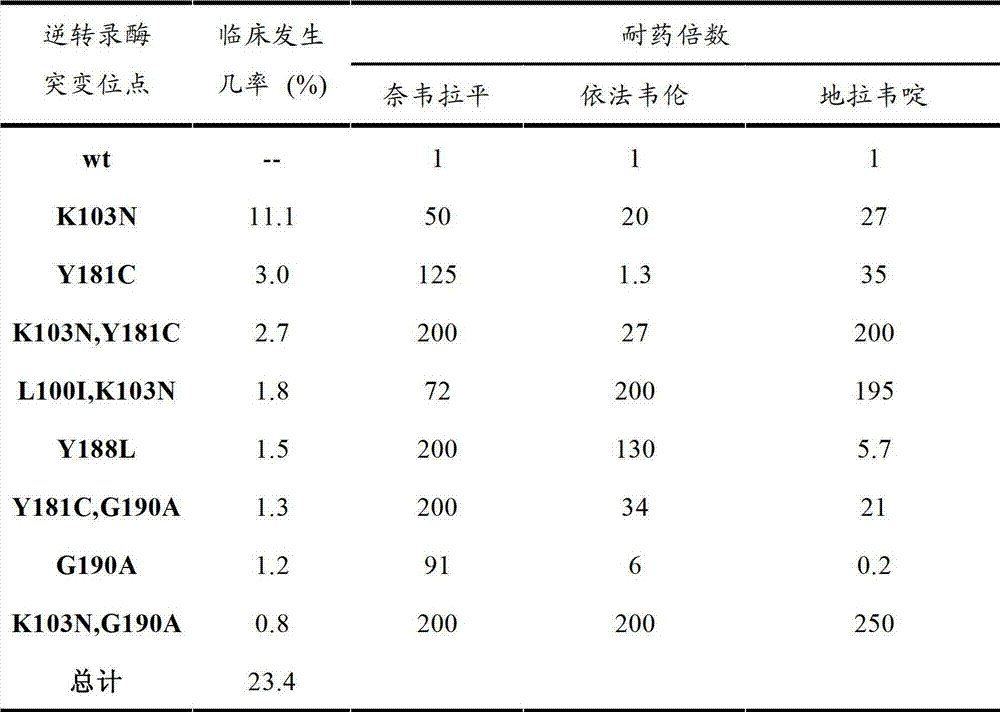
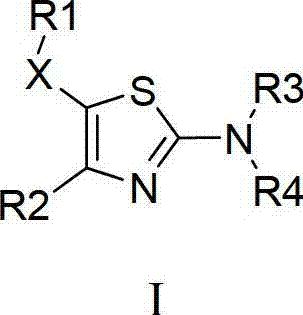
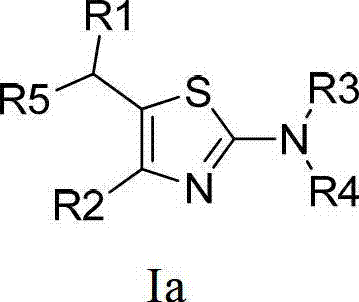
Thiazole compound capable of restraining human immunodeficiency virus (HIV) replication and effective against drug-resistant HIV virus strains and preparation method and purpose thereof
A compound and medicinal salt technology, applied in the field of thiazole compounds, which can inhibit HIV replication and are effective against drug-resistant HIV strains, and its preparation and application, can solve problems such as treatment failure and mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] The preparation of embodiment 1N-benzyl-5-benzyl-4-(4-methoxyphenyl)-thiazol-2-amine
[0226]
[0227] Step 1 Preparation of (E)-1-(4-methoxyphenyl)-3-phenylprop-2-en-1-one
[0228] Put 0.57g (3.8mmol) of p-methoxyacetophenone in a 25mL three-necked flask, add 6mL of absolute ethanol, add 5.7mL of sodium hydroxide solution, stir for 5min, and add 0.51g of (3.8mmol) benzaldehyde, kept in ice-water bath for 4h, TLC monitored the completion of the reaction, filtered, washed with water, and recrystallized from ethanol to obtain 0.81g of light yellow solid with a yield of 90.0%.
[0229] Step 21- Preparation of (4-methoxyphenyl)-3-phenylpropan-1-one
[0230] Dissolve 3.66g (15.39mmol) of (E)-1-(4-methoxyphenyl)-3-phenylprop-2-en-1-one prepared in step 1 in 80mL of ethyl acetate, add catalyst Measure Pd / C, after catalytic hydrogenation for 2 hours, filter, and rotary steam under reduced pressure to obtain 3.68g of transparent oily substance, yield 99.6%, m.p.96.5-97.2°C....
Embodiment 2
[0239] The preparation of embodiment 2N-(4-methoxybenzyl)-5-benzyl-4-(4-methoxyphenyl)-thiazol-2-amine
[0240]
[0241] Adopt the preparation method of embodiment 1 step 5 and step 6, 5-benzyl-4-(4-methoxyphenyl)-thiazol-2-ylamine and p-methoxybenzene prepared by embodiment 1 step 4 Formaldehyde was used as a raw material to obtain a white solid with a yield of 85.0% and m.p.151.2.7-152.4°C. 1 H NMR (CDCl 3 ,300MHz)δ:3.80(s,3H,OCH 3 ),3.81(s,3H,OCH 3 ),4.00(d,2H,J=6.0Hz,ArCH 2 ),4.35(s,2H,ArCH 2 ),5.35(br,1H,NH),6.85~6.91(m,4H,ArH),7.21~7.33(m,7H,ArH),7.49~7.52(m,2H,ArH); ESI-MS m / z :417([M+H] + ).
Embodiment 3
[0242] The preparation of embodiment 3N-(3-methoxy-4-hydroxybenzyl)-5-benzyl-4-(4-methoxyphenyl)-thiazol-2-amine
[0243]
[0244] Adopt the preparation method of embodiment 1 step 5 and step 6, 5-benzyl-4-(4-methoxyphenyl)-thiazol-2-ylamine and 4-hydroxyl-3 prepared by embodiment 1 step 4 -Methoxybenzaldehyde was used as a raw material to obtain a white solid with a yield of 73.0%, m.p.137-138°C. 1 H NMR (CDCl 3 ,600MHz)δ:3.81(s,3H,OCH 3 ),4.10(s,2H,ArCH 2 ),4.39(s,2H,ArCH 2 ),5.80(br,1H,NH),6.90(d,J=8.4Hz,2H,ArH),7.02(d,J=8.4Hz,2H,ArH),7.23~7.33(m,7H,ArH), 7.50(d,J=7.5Hz,2H,ArH);ESI-MS m / z:433([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com