A fully-closed pipeline protein purification system and its application in the preparation of sterile pyrogen-free protein drugs
A protein purification and fully enclosed technology, applied in the field of protein purification, can solve the problems of restricting protein drugs, and achieve the effect of reasonable design, tight connection and easy expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: The composition and assembly of a fully closed pipeline protein purification system
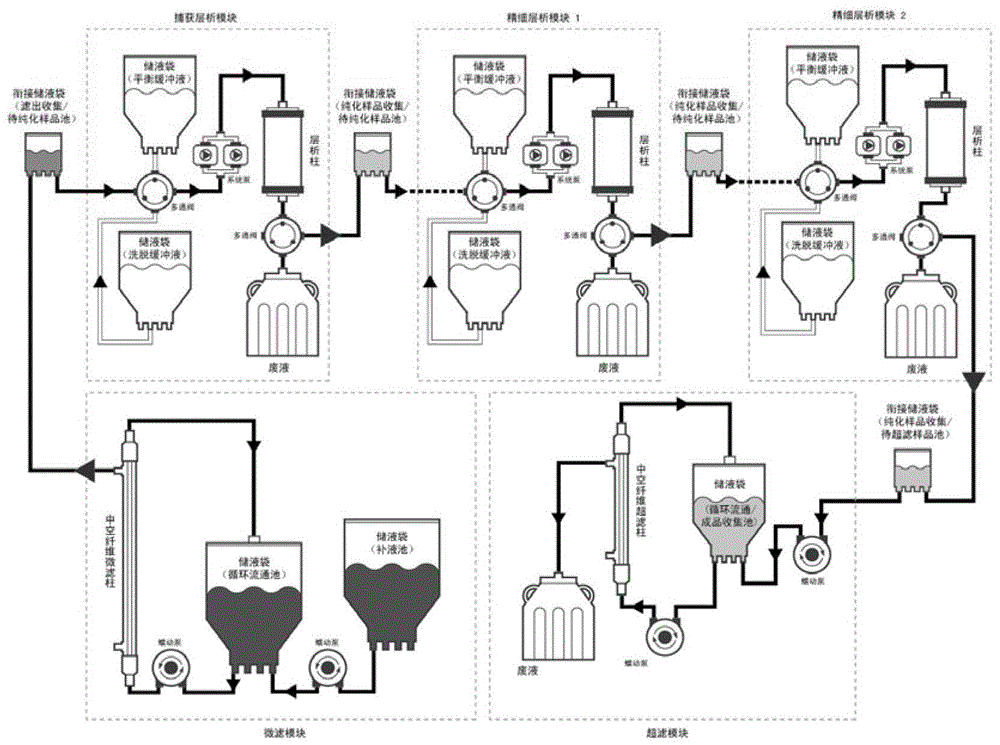
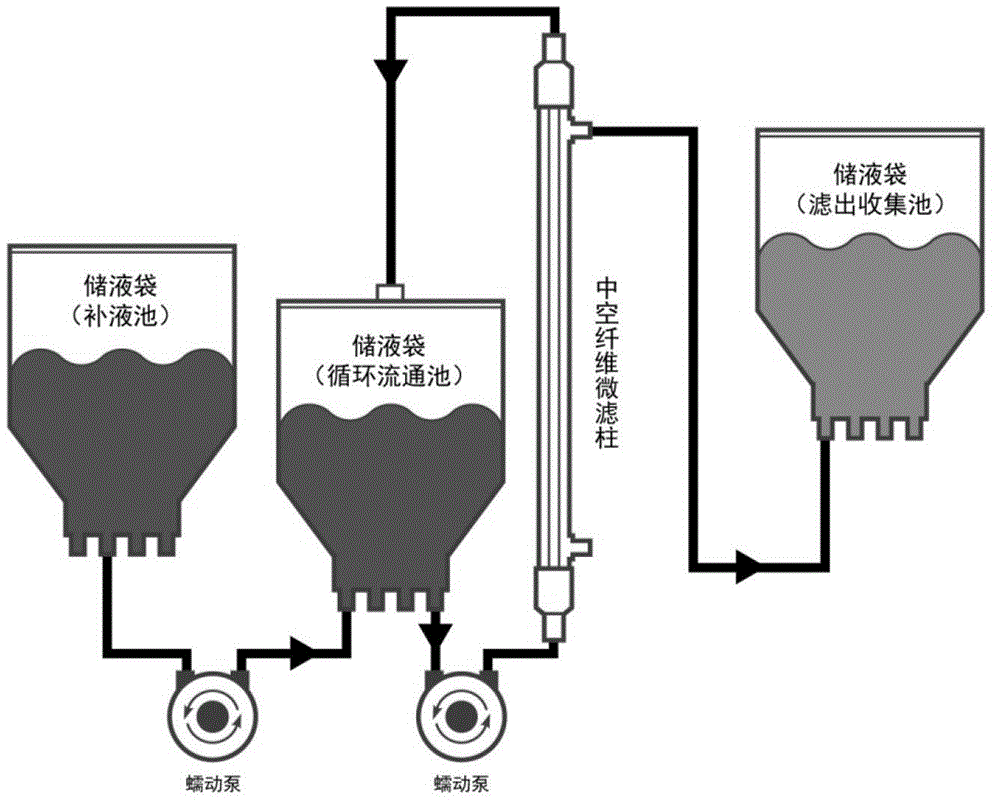
[0063] The fully-enclosed pipeline protein purification system of the present invention is aimed at the post-expression separation and purification process of soluble secreted and expressed genetically engineered proteins, and includes hardware modules for respectively performing process steps such as microfiltration clarification, chromatography purification, ultrafiltration concentration, and dosage forms. . The technical key point and innovation point of the present invention is to assemble the process steps and hardware that were separated and semi-closed in the past into a fully closed process and hardware system that can be operated continuously in sequence through appropriate connection tools. like figure 1 As shown, the composition and assembly of the purification system includes the following specific contents:
[0064] First, the hardware module for performing th...
Embodiment 2
[0070] Example 2: A fully closed pipeline system suitable for humanized anti-CD146 monoclonal antibody and a pilot-scale aseptic and pyrogen-free purification preparation process
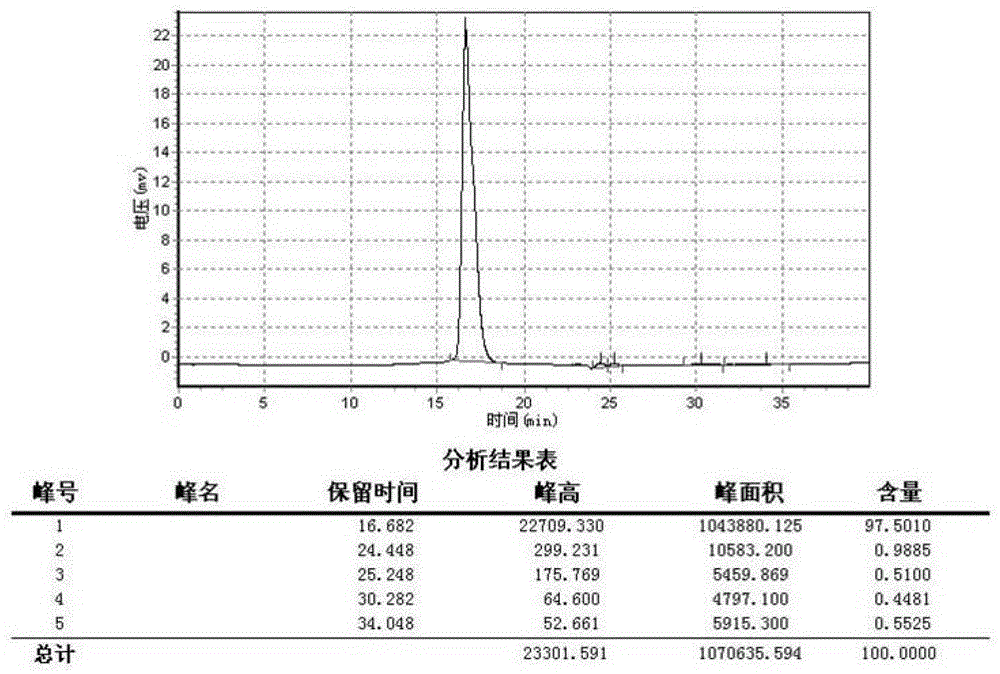
[0071] Figure 5 It is a schematic diagram of the assembly of the fully closed pipeline protein purification system combined with the humanized anti-CD146 monoclonal antibody purification process described in the present invention. As shown in the figure, the system is in the order of process, followed by a microfiltration module based on a hollow fiber column with a pore size of 0.45 μm, and an affinity chromatography medium based on Protein A ligand (GE healthcare Bio-Sciences Corp. Product Code: 17-5474-02) Capture chromatography module, fine chromatography module based on Superdex200 (GEhealthcareBio-SciencesCorp.ProductCode: 17-1043-01) gel filtration chromatography medium and ultrafiltration module based on 50kD molecular weight cut-off hollow fiber column; The method described in Embodiment ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com