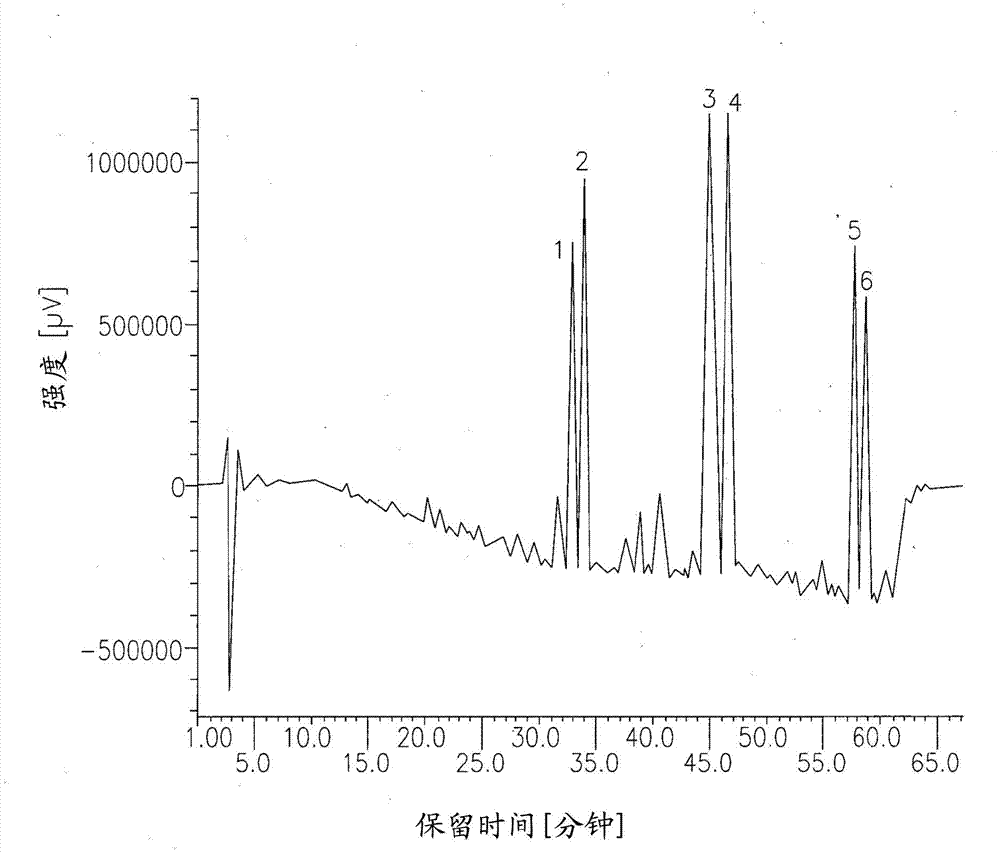
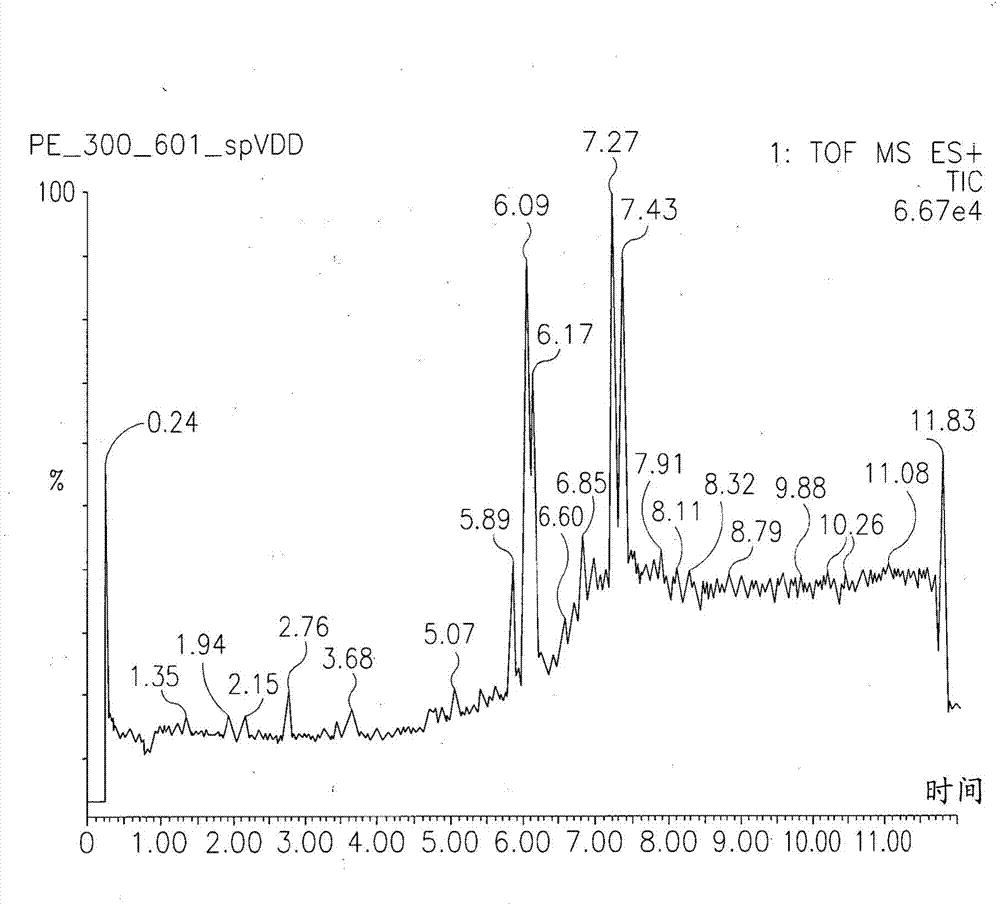
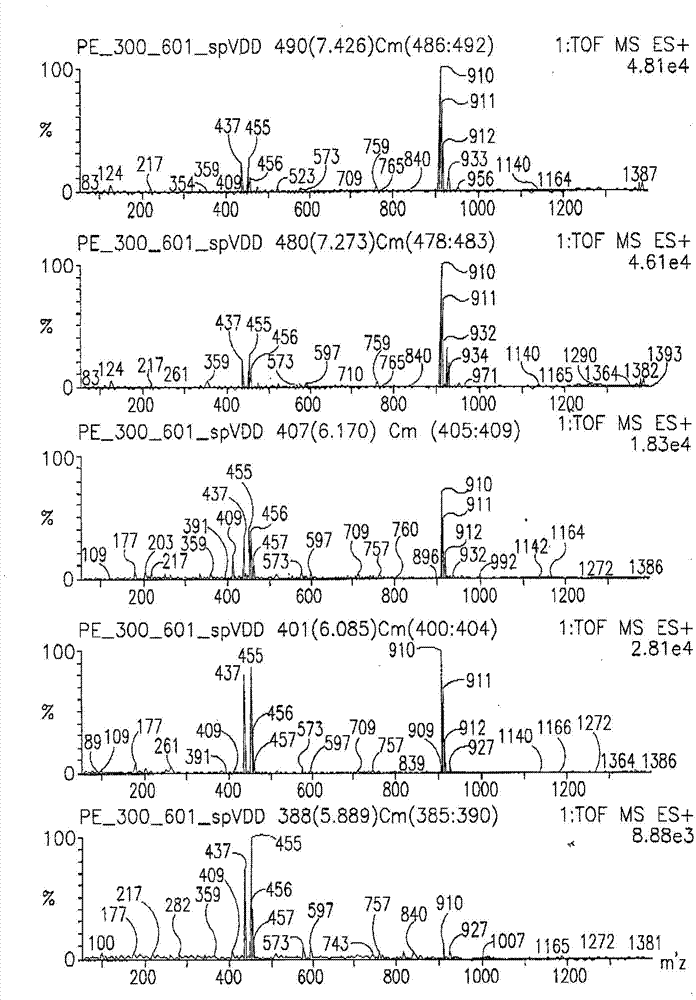
Compositions comprising acidic extracts of mastic gum
A composition, the technology of frankincense, applied in the direction of drug combination, active ingredients of anhydride/acid/halide, medical preparations containing active ingredients, etc., to achieve the effect of promoting wound healing and prolonging life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0231] Commercial preparations of mastic are available, for example, from Chios Gum Mastic Growers Association or G. Baldwin & Co., U.K.
[0232] Analytical methods used to determine the precise chemical composition of the isolated acidic fraction of boswellia obtained include nuclear magnetic resonance (e.g. 1 HNMR and 13 CNMR), viscometry, various mass spectrometry methods (e.g. MALDI-TOF), HPLC, combined methods such as liquid chromatography-mass spectrometry (LC-MS), UV-VIS spectrometry, IR and FT-IR spectroscopy Assays and other methods known in the art.
[0233] The method for obtaining the isolated acidic fraction of Boswellia can be as described below. By way of overview, the collected plant matter, such as frankincense, is mixed in a suitable vessel with a suitable solvent, usually a polar solvent. Suitable polar solvents include, for example, alcohols, ethers, esters, amides, aldehydes, ketones, nitriles, and combinations thereof.
[0234] Specific examples of po...
Embodiment 1
[0325] Example 1. Preparation of the Isolated Acidic Fraction of Boswellia
[0326] Boswellia (10 g) was mixed with absolute ethanol (200 ml) and the mixture was allowed to stand overnight. The mixture was shaken at 150 rpm for about 15 minutes, any larger insoluble particles were allowed to settle for 20 minutes, and the ethanol was transferred to a new flask. The residue was shaken at 200 rpm for 10 min with a fresh portion of absolute ethanol (150 ml). This ethanol fraction was mixed with the first fraction. The process was repeated with another 150 ml portion of absolute ethanol, and this fraction was mixed with the first two ethanol fractions. Subsequently, ethanol was removed in vacuo using a rotary evaporator (water bath temperature 30 °C). Hexane (300 ml) was added to the residue, and the mixture was shaken at 150 rpm for two hours. Leave overnight in a closed flask to complete dissolution of soluble material and precipitation of any insoluble material, then tran...
Embodiment 2
[0328] Example 2. Isolated Acidic Fraction (RPh-Ac) of Boswellia in USP / NF Grade Cottonseed Oil Preparation of 5% (w / w) composition
[0329] To 1 gram of the isolated acidic fraction obtained from Example 1 was added 19 grams of cottonseed oil (USP / NF) and the mixture was shaken at 150 rpm until a clear and homogeneous composition was obtained (about 2 hours).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com