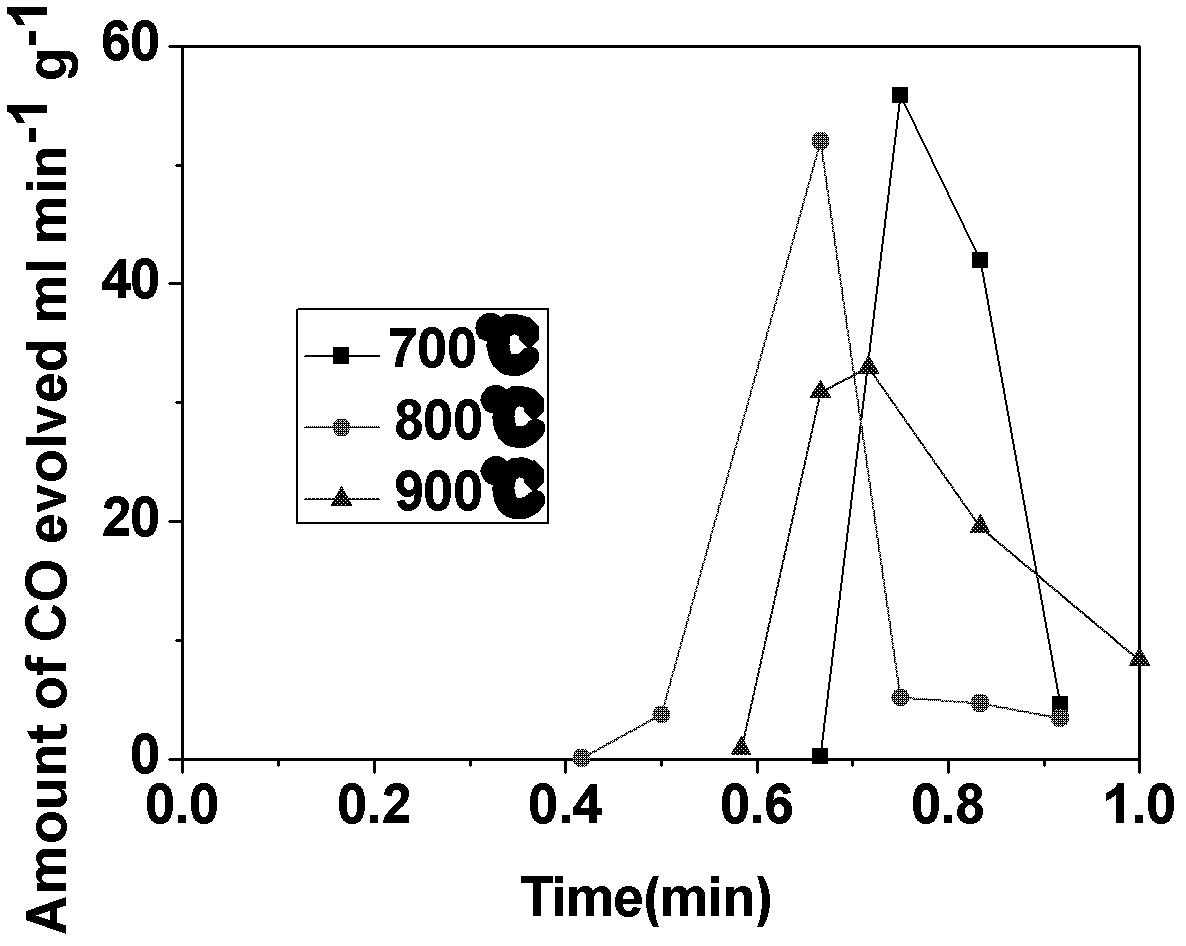
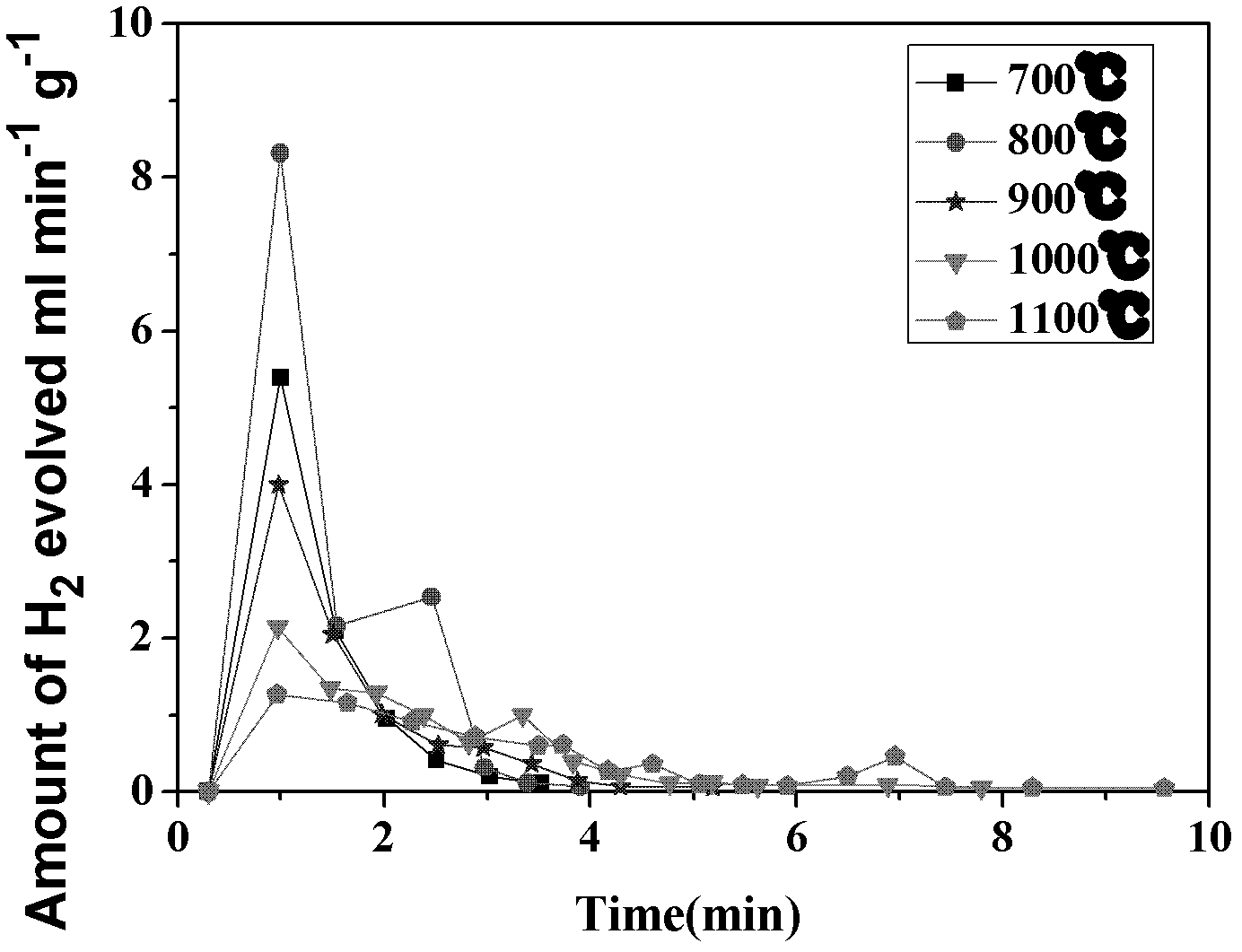
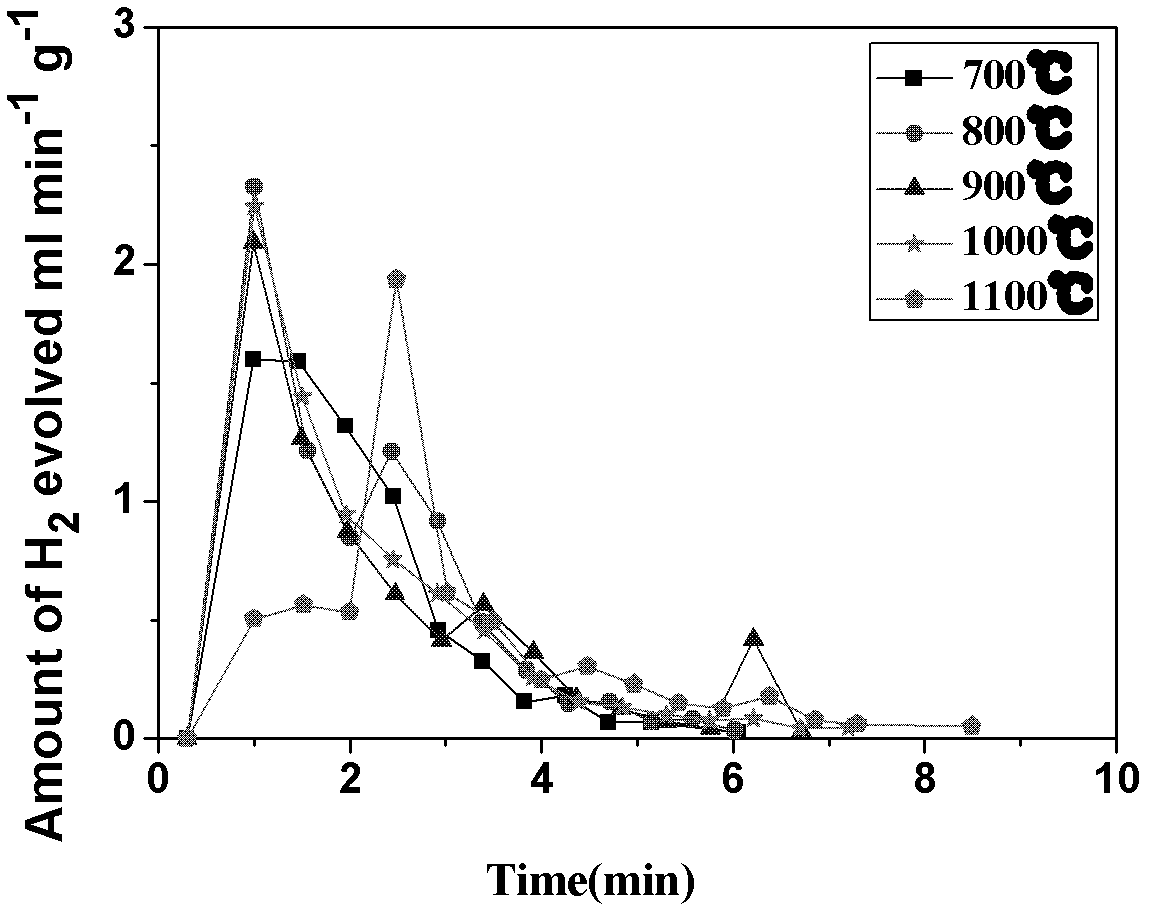
Application of CeO2-based active material to two-step thermochemical cycle decomposition of H2O and/or CO2
A thermochemical cycle and active material technology, applied in the field of preparing H2/CO, which can solve the problems of poor cycle performance, high reduction temperature of metal oxides, and low amount of oxygen vacancies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] a: Weigh 5.4822g of cerium ammonium nitrate (10mmol) and dissolve it in 75ml of deionized water. After stirring at room temperature for 30min, add 3.1ml (30mmol) of diethylenetriamine DETA (H 2 NCH 2 CH 2 NHCH 2 CH 2 NH 2 ), continue to stir for 30 minutes, pour into a 100ml capacity hydrothermal kettle, and treat at 200°C for 24h; after cooling, centrifuge, dry, and roast at 600°C for 4h.
[0054] When M=La 3+ , Y 3+ 、Sm 3+ When one or more of them, Ce x m 1-x o 2-δ The preparation process of solid solution is as follows:
[0055] Weigh 5.2081-4.660g (NH 4 ) 2 Ce(NO 3 ) 6 , and 0.2165-0.6498gLa (NO 3 ) 3 .6H 2 O, 0.1915-0.5745g Y (NO 3 ) 3 .6H 2 O, 0.1682-0.5046g Sm(NO 3 ) 3 .6H 2 One or more than two kinds of O, dissolved in 75ml deionized water, stirred at room temperature for 30min, then added 3.1ml (30mmol) diethylenetriamine DETA (H 2 NCH 2 CH 2 NHCH 2 CH 2 NH 2 ), continue to stir for 30 minutes, pour into a 100ml capacity hydrotherma...
Embodiment 2
[0060] Weigh 0.5g DETA as a precipitant hydrothermally synthesized CeO 2 Sample (marked as: CeO 2 -1), placed in a reaction tube, the Ar gas flow rate is 100ml / min, the temperature is raised to 1500°C at 5°C / min, the temperature is treated for 40min, the temperature is lowered to 800°C, the temperature is adjusted, the Ar flow rate is adjusted to 400ml / min, and the 100°C The preheated water vapor is brought into the reaction tube, the volume ratio of Ar gas to water vapor is 1:1, and the reaction is completed within 10 minutes. Income O 2 The quantities are listed in Table 1.
Embodiment 3
[0062] Same as Example 2, except that the temperature for decomposing water is 700°C, 900°C, 1000°C, 1100°C, 1200°C;
[0063] When the temperature of decomposed water is 700°C, 900°C, 1000°C or 1100°C, the reaction should be completed within 10 minutes;
[0064] When the temperature of decomposed water is 1200°C, the reaction is completed for 2 hours, and the cycle reaction is repeated for 3 times.
[0065] The results are shown in Table 2, and the cycle results are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com