A kind of method for preparing o-veratrol using veratrol as raw material
A technology of o-veratraldehyde and veratrol, which is applied in the field of preparation of chemical drug intermediates, can solve the problems of environmental pollution, high purchase cost, and mild conditions, and achieve reduced environmental toxicity, good reaction yield, and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
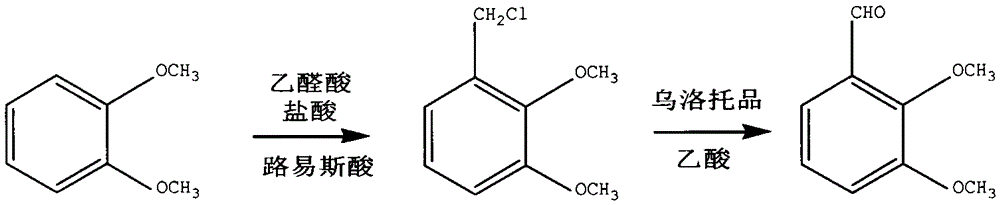
[0027] Add 13.8g of veratrole into a 250mL glass three-necked flask, start stirring, add 7g of sodium trifluoromethanesulfonate as catalyst, add 16mL of 35% hydrochloric acid solution dropwise, add 8ml of glyoxylic acid dropwise, heat up to 70°C after adding, and react 8h. After the reaction, the reaction liquid was cooled, and 100 ml of water and 100 ml of petroleum ether were added for extraction, and the lower aqueous layer was separated. Add 8ml of acetic acid dropwise to the upper layer, add 20g of urotropine, stir and react at room temperature for 2h, wash the reaction solution with water, let it stand for layering, take the organic phase and concentrate under reduced pressure, and after cooling, o-veratraldehyde crystals are obtained. Yield 85%, purity 95.0% (GC).
Embodiment 2
[0029] Add 13.8g of veratrole into a 250mL glass three-necked flask, start stirring, add 7g of sodium trifluoromethanesulfonate as catalyst, add 16mL of 35% hydrochloric acid solution dropwise, add 8ml of glyoxylic acid dropwise, heat up to 70°C after adding, and react 5h. After the reaction, the reaction liquid was cooled, and 100 ml of water and 100 ml of petroleum ether were added for extraction, and the lower aqueous layer was separated. Add 8ml of acetic acid dropwise to the upper layer, add 20g of urotropine, stir and react at room temperature for 2h, wash the reaction solution with water, let it stand for layering, take the organic phase and concentrate under reduced pressure, and after cooling, o-veratraldehyde crystals are obtained. Yield 82%, purity 65.5% (GC).
Embodiment 3
[0031] Add 13.8g of veratrole into a 250mL glass three-necked flask, start stirring, add 7g of catalyst sodium trifluoromethanesulfonate, add 16mL of 35% hydrochloric acid solution dropwise, add dropwise 8ml of glyoxylic acid, heat up to 50°C after the addition, and react 10h. After the reaction was completed, the reaction liquid was cooled, and 50 ml of water and 50 ml of petroleum ether were added for extraction, and the lower aqueous layer was separated. Add 8ml of acetic acid dropwise to the upper layer, add 20g of urotropine, stir and react at room temperature for 2h, wash the reaction solution with water, let it stand for layering, take the organic phase and concentrate under reduced pressure, and after cooling, o-veratraldehyde crystals are obtained. Yield 85%, purity 94.8% (GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com