Application of quercetin derivative in preparation of antitumor medicine
A tumor drug and drug technology, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of poor solubility of quercetin, large intermolecular attraction, quercetin activity research and clinical application limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
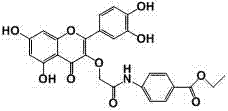
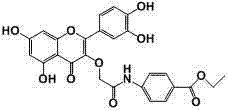
[0014] Example 1 Structural characterization of compound QB
[0015] M.p: 294.9~296.8 ℃;
[0016] 1 H NMR (400 MHz, DMSO) δ 12.43 (s, 1H,5-OH), 10.90 (s, 1H,7-OH), 10.59 (s, 1H,NH), 9.80 (s, 1H, 4′-OH ), 9.45 (s, 1H, 3′-OH), 7.95 (d, J = 8.8 Hz, 2H, 2′-H, 6′-H), 7.83 (d, J = 8.8 Hz, 2H,2×ph -H), 7.61 – 7.54 (m, 2H, 2×ph-H), 6.91 (d, J = 9.0 Hz, 1H, 5′-H), 6.46 (d, J = 2.0 Hz, 1H,8-H ), 6.24 (d, J = 2.0 Hz, 1H,6-H), 4.65 (s, 2H, COCH 2 ), 4.30 (q, J = 7.1 Hz, 2H,CH 3 CH 2 ), 1.32 (t, J = 7.1 Hz, 3H,CH 3 ).
[0017] 13 C NMR (101 MHz, DMSO) δ 178.04, 167.82, 165.77, 164.88, 161.57, 156.87, 156.30, 149.43, 145.81, 143.09, 137.19, 130.72, 125.16, 121.58, 120.93, 119.37, 116.26, 116.01, 104.43, 99.27, 94.24 , 71.67, 60.95, 14.68.
Embodiment 2
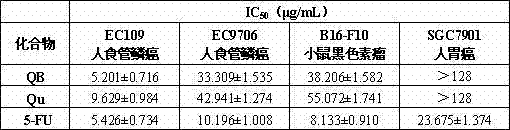
[0018] Example 2 Antitumor Activity Test
[0019] 2.1 Cell lines and reagents
[0020] Human esophageal squamous cell carcinoma cells EC109, human esophageal squamous cell carcinoma cells EC9706, human gastric cancer cells SGC7901 and mouse melanoma cells B16-F10 were purchased from the Cell Bank of Shanghai Institute of Biological Sciences, Chinese Academy of Sciences.
[0021] Dimethyl sulfoxide (DMSO), Sigma
[0022] Tetramethylazolazolium blue (MTT), Sigma
[0023] RPMI-1640 medium, Thermo Fisher Biochemicals (Beijing) Co., Ltd.
[0024] Fetal bovine serum, Thermo Fisher Biochemicals (Beijing) Co., Ltd.
[0025] Trypsin, Hangzhou Jinuo Biomedical Technology Co., Ltd.
[0026] Medium: Take RPMI-1640 medium, add 10% fetal bovine serum, penicillin 100U / mL, streptomycin 100μg / mL, mix well, store at 4°C for later use.
[0027] Cell cryopreservation solution: Add DMSO to the medium containing 10% fetal bovine serum to make the DMSO concentration 10%.
[0028] Trypsin d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com