Parapoxvirus vectors containing rabies virus antigen
A rabies virus, pox virus technology, applied in the application field of recombinant parapox virus in diagnostics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0116] The preparation of parenteral formulations under sterile conditions (eg, by lyophilization) is readily accomplished using standard pharmaceutical techniques well known to those skilled in the art.
[0117] The recombinant parapoxviruses and immunogenic compositions and vaccines described herein can be used in the preparation of medicaments for vaccinating animals against rabies.
[0118] The present invention provides methods for determining the source of a parapoxvirus present in an animal.
[0119] Vaccination with the DIVA vaccine, which distinguishes infected animals from vaccinated animals, provides a way to determine the source of parapoxviruses present in animals. This differentiation can be achieved via any of a variety of diagnostic methods including, but not limited to, ELISA, Western blot, and PCR. These and other methods are readily recognized and understood by those of ordinary skill in the art.
[0120] The parapoxviruses described herein can be distingu...
Embodiment 1
[0127] Example 1. Production of rabies G protein expressing recombinant virus D1701-V-RabG
[0128] Recombinantly produced ovine parapoxviruses containing the gene encoding the G protein of RV were generated. The expression of the G protein and the immunostimulatory and protective properties of the recombinant virus against RV were assessed.
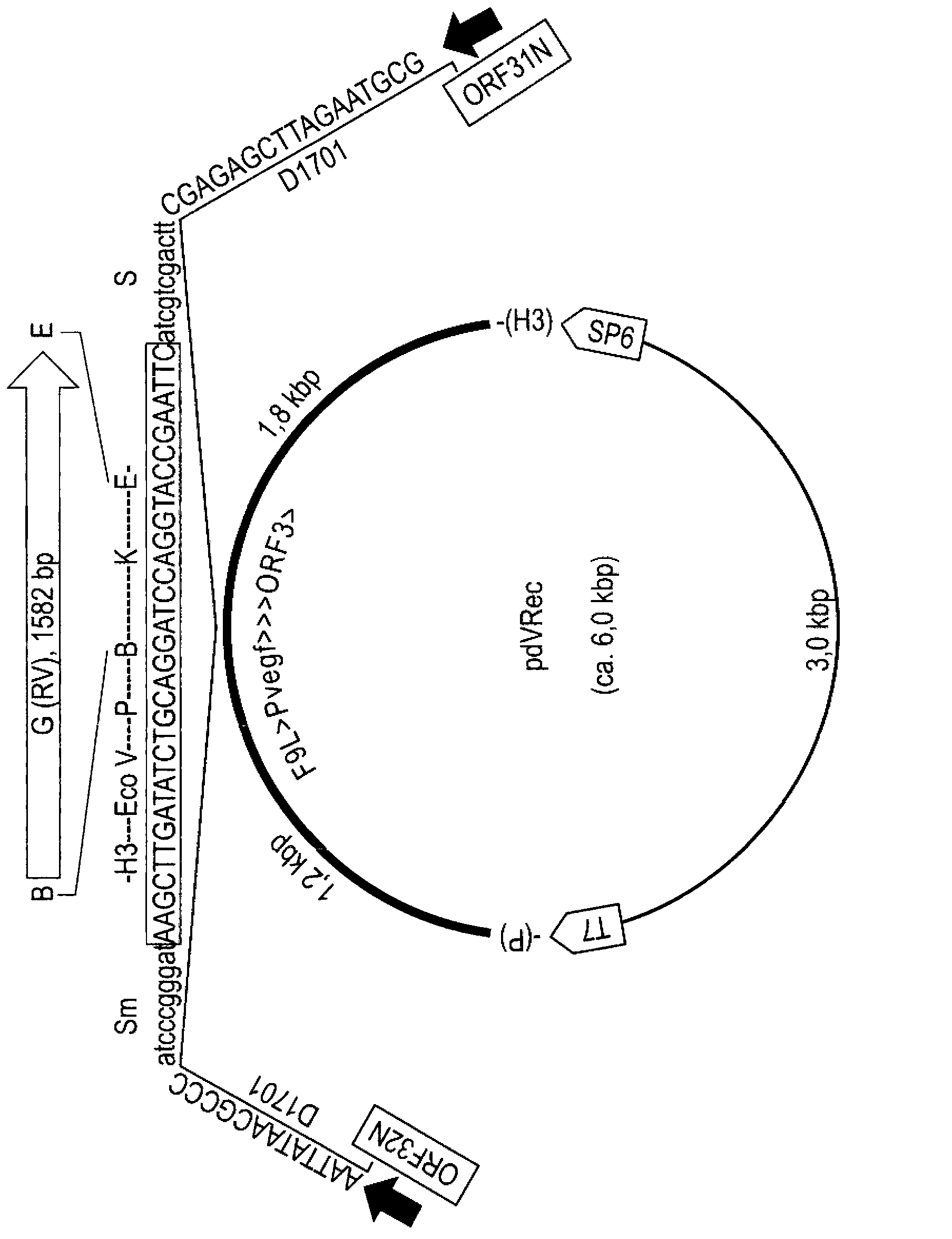
[0129] Construction of the transfer plasmid
[0130] For the production of recombinant ovine parapoxvirus D1701-V-RabG, the ovine parapoxvirus (PPVO) vector system was used (US Pat. J. Virol. 77, 9312-9323; Henkel et al., 2005, J. Virol. 79, 314-325). The rabies virus G protein gene was chemically synthesized (Blue HeronBiotech; USA) and provided in a pUC plasmid. The intact G gene was separated into a BamHI-EcoRI DNA fragment with a size of 1,582 bp by agarose gel (0.8%, weight / volume ratio) electrophoresis and purified by Qiaex II gel extraction kit (Qiagen; Germany). Plasmid pdV-Recl (Fischer et al., 2003) was double digested wi...
Embodiment 2
[0138] Example 2. Characterization of D1701-V-RabG
[0139] Preparation of virus stock solution
[0140] In order to obtain a high-titer recombinant virus stock solution, 10-20 T150 culture flasks (Greiner; Germany) were simultaneously infected at an MOI of 0.5. After 3 days, approximately 80% cytopathic effect (CPE) was observed, and cells and supernatants in all flasks were collected for centrifugation (13,000 rpm at 4°C for 2 hours). Carefully remove the supernatant, and dissolve the virus pellet in 1ml~2ml PBS overnight at 4°C. The virus suspension was completely dispersed by ultrasound (Soniccell disruptor, Branson; Germany) using three 10-second pulses (100 W) on ice (10-second rest between each pulse), followed by centrifugation (500 × g ~ 700 × g, 10 min, 4°C) to remove cell debris. The supernatant was stored on ice while the cell pellet was resuspended in 1.0 ml PBS and sonicated on ice (2 x 20 sec with 10 sec rest in between, then 1 x 30 sec). After centrifugat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com