Artificial biological tendon and preparation method thereof
A biological and artificial technology, applied in the field of artificial biological tendon and its preparation, can solve the problems that affect the product's anti-infection ability, tissue regeneration ability, product instability, and damage to the three-dimensional structure, so as to increase the DNA removal process, ensure stability and Uniformity, low immunogenicity and high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of 8-layer artificial biological tendon
[0043] The preparation method is as follows:
[0044] (1) Determination of animal source and pretreatment and initial washing of small intestine tissue
[0045] The Chinese inbred Wuzhishan minipig was selected as the animal source, and the animal species was determined using the method specified in the patent ZL200510008994.2. The small intestine tissue of the freshly slaughtered Chinese inbred Wuzhishan minipig was cleaned, and the small intestinal submucosa was separated and used Rinse with water for injection 3 times.
[0046] (2) Virus inactivation
[0047] The peracetic acid-ethanol solution method is used to inactivate the virus. This step is carried out in a constant temperature ultrasonic cleaner that can shake the cleaning tank. The volume percentage of peracetic acid is 0.05~0.2% (preferably 0.1%). The time is 1~2h (preferably 1h), the temperature range is 4~40°C, and then washed 2~5 times...
Embodiment 2
[0056] Embodiment 2: Physical and chemical properties, histology, growth factors and biological performance detection of the material prepared in embodiment 1
[0057] 1. The physical properties of the prepared 8-layer material are tested, and the testing items include porosity measurement, suture retention, tensile strength, and burst strength.
[0058] 1) Porosity measurement: Mercury porosimetry was used to measure the porosity of the material, and compared with and compared with Biodesign Surgisis products. Results: The porosity of the sample provided in Example 1 was 87.8±2.51, and the porosity of the Biodesign Surgisis product was 78.3±6.38.
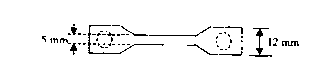
[0059] 2) Suture retention test: method: use 2-0 surgical sutures or stainless steel wires of the same diameter to suture 2mm inside the edge of one end of the artificial biological tendon, and fix the suture or stainless steel wire and the other end of the artificial biological tendon on the On the tension meter, stretch at a spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Burst strength | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com