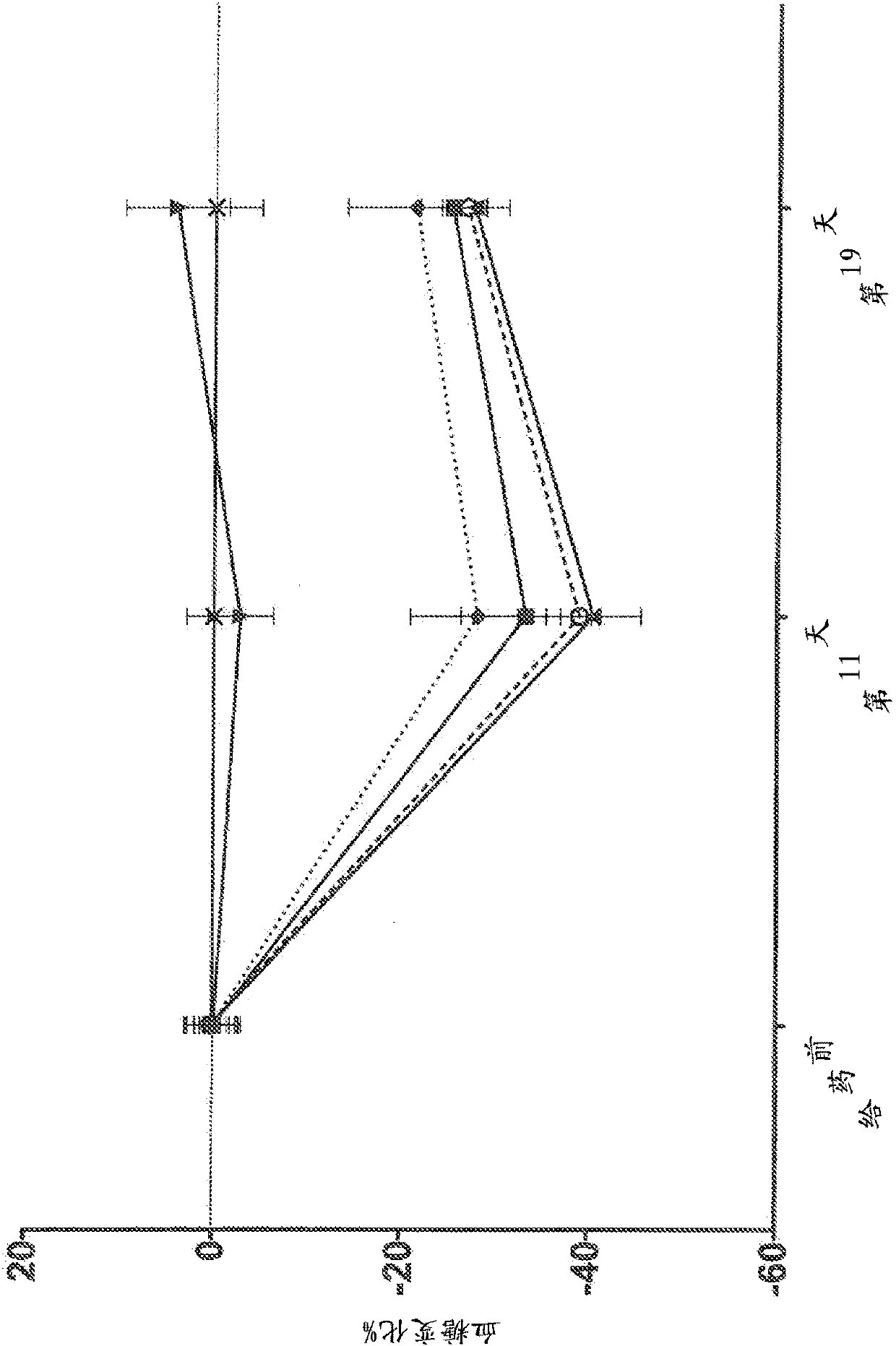
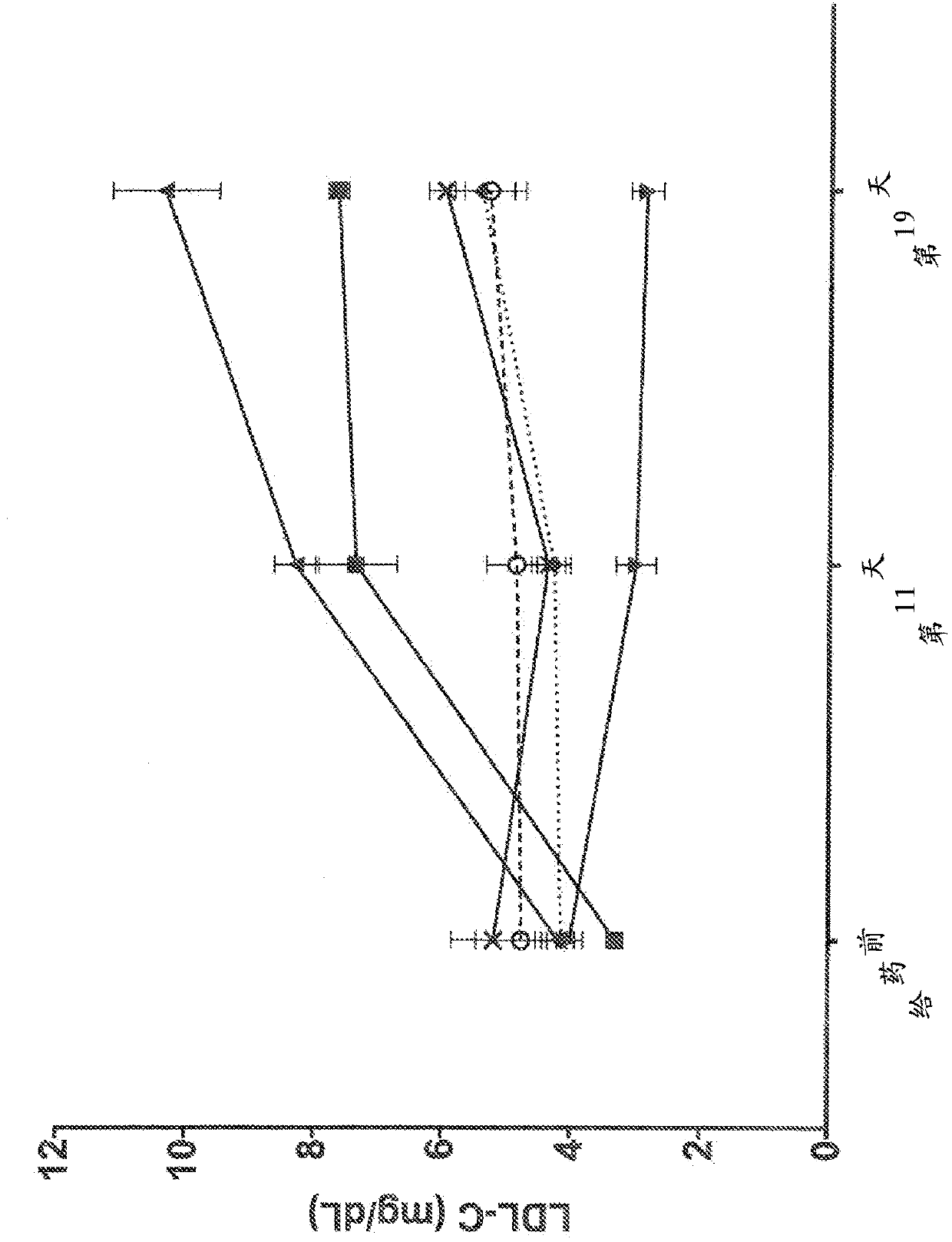
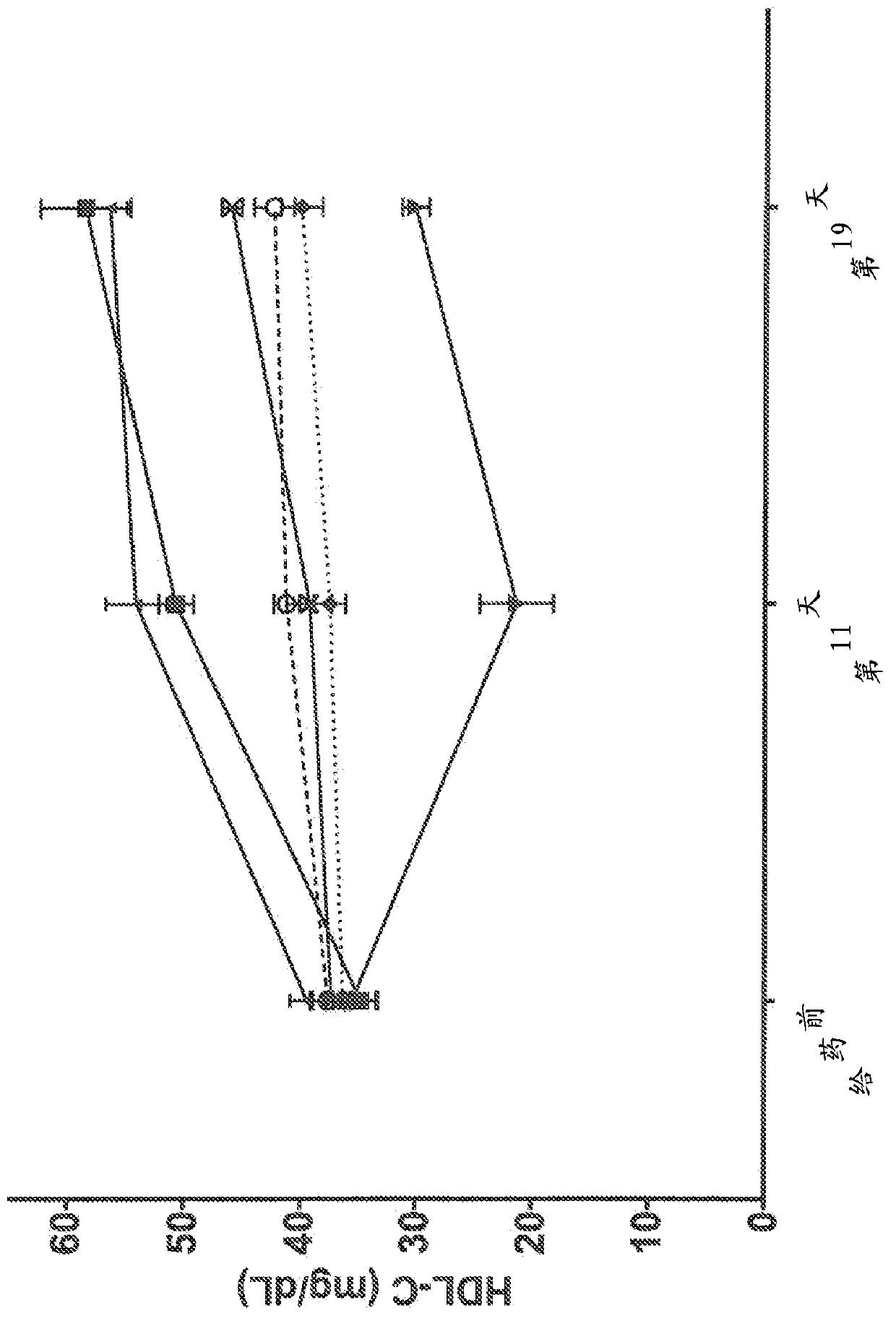
Human antibodies to the glucagon receptor
A glucagon and antibody technology, applied in the direction of antibodies, antibody medical components, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of not recognizing mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] Production of Human Antibodies
[0130] Methods for producing human antibodies in transgenic mice are known in the art. Any such known methods can be used in the context of the present invention to prepare human antibodies that specifically bind to human GCGR.
[0131] Use VELOCIMMUNE TM technology (see, for example US6,596,541, Regeneron Pharmaceuticals, ) or any other known method for producing monoclonal antibodies, initially isolating a high affinity chimeric antibody to GCGR having human variable regions and mouse constant regions. Techniques include the generation of transgenic mice having a genome comprising human heavy and light chain variable regions operably linked to endogenous mouse constant region loci such that the mice respond to antigenic stimulation to produce human variable regions and Antibodies against mouse constant regions. DNA encoding the variable regions of the heavy and light chains of the antibody is isolated and operably linked to DNA e...
Embodiment 1
[0205] Example 1 Production of Human Antibodies to Human GCGR
[0206] Antibodies to hGCGR can be raised using immunogens including any of the following. For example, in certain embodiments, cells expressing hGCGR are used as immunogens to generate antibodies to hGCGR. Furthermore, in certain embodiments, DNA encoding hGCGR is used as an immunogen to prepare antibodies of the invention. Furthermore, in certain embodiments, a peptide comprising the amino acid sequence of the N-terminal domain of hGCGR is used as an immunogen to generate antibodies to human GCGR. Furthermore, in certain embodiments, a peptide comprising the amino acid sequence of any of the extracellular loop regions ECl, EC2, or EC3 of hGCGR can be used as an immunogen to generate antibodies to human GCGR. Cells, DNA or peptides used as immunogens as described above are administered directly to the A mouse comprising DNA encoding human immunoglobulin heavy chain and kappa light chain variable regions. Anti...
Embodiment 2
[0209] Example 2 Amino Acid Sequences of Heavy Chain and Light Chain Variable Regions
[0210] Table 1 presents the heavy chain and light chain variable region amino acid sequence pairs of selected anti-GCGR antibodies and their corresponding antibody identifiers. Antibody names with the same numerical value but different letter suffixes N, B, or P refer to antibodies that have heavy and light chains that contain the same CDR sequences but sequence changes in regions outside the CDR sequences (i.e., within the framework regions). chain of antibodies. Thus, N, B and P variants of a particular antibody have identical CDR sequences within their heavy and light chain variable regions, but differ from each other within their framework regions.
[0211] Table 1
[0212]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com