Novel VQ polypeptide radioactive medicine and preparation method thereof
A radiopharmaceutical and radionuclide technology, applied in the field of radiopharmaceuticals with novel heptapeptide sequences and their preparation, can solve the problem of low early diagnosis rate of colon cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
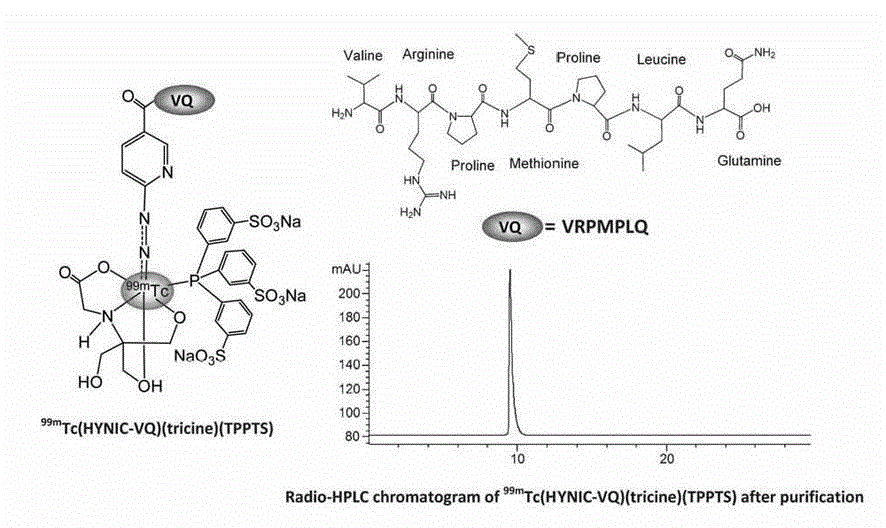
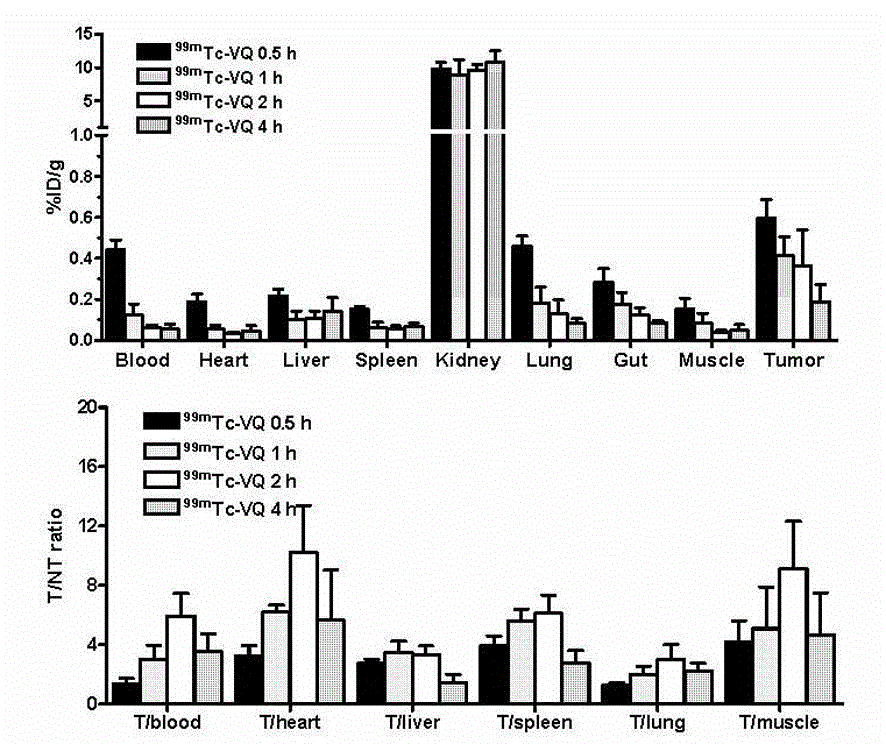
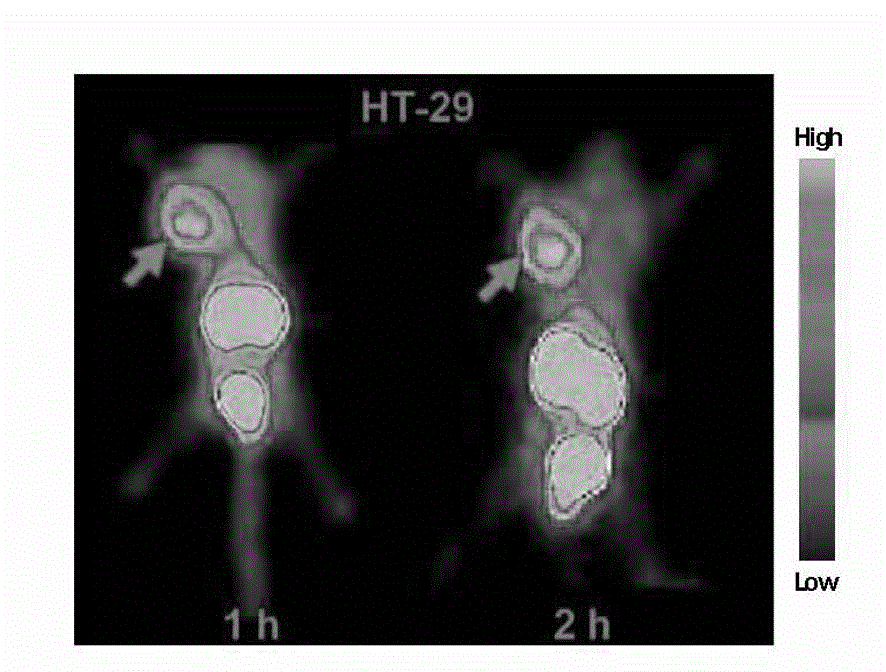
[0034] A VQ polypeptide radiopharmaceutical, including VQ polypeptide, a bifunctional chelator (Chelator) and a radionuclide (Nuclide), the VQ polypeptide is a linear hepeptide, and the linear hepeptide sequence is arginine-arginine- Proline-Methionine-Proline-Leucine-Glutamine VRPMPLQ (Val-Arg-Pro-Met-Pro-Leu-Gln, VQ), the radionuclide passes through a bifunctional chelating agent The VQ linear heptapeptide is labeled, the VQ polypeptide radiopharmaceutical is Nuclide-Chelator-VQ, and the VQ polypeptide radiopharmaceutical is a colorless transparent liquid injection.
[0035] Further, the bifunctional chelating agent is HYNIC (hydrazinonicotinamide). In the present invention, HYNIC is used as a bifunctional chelating agent, and tricine (trimethylolglycine) and TPPTS (trisodium triphenylphosphine-3,3',3''-trisulfonate) are used as a synergistic complex. Body so that " 99m "Tc-HYNIC core" has better stability in vivo and in vitro.
[0036] Further, the radionuclide is 99m Tc, the ...
Embodiment 2
[0045] This embodiment is an improvement made on the basis of Embodiment 1. For the same parts in this embodiment as Embodiment 1, please refer to the content disclosed in Embodiment 1 for understanding. The content disclosed in Embodiment 1 should also be regarded as this embodiment. The content is not repeated here.
[0046] a. Preparation of HYNIC-VRPMPLQ (HYNIC-VQ)
[0047] HYNIC-NHS (2 mg, 4.8 μmol) and VQ (1.2 mg, ~1.2 mmol) dissolved in 2 mL of N,N-Dimethylform amide (DMF, N,N-dimethylformamide) and H 2 O mixture (1:1 = v:v), adjust the pH to 8.5-9.0 with 0.1 N NaOH, and stir overnight at room temperature. The product was separated and purified by HPLC method 1, and the fraction with a retention time of 13.7 minutes was collected. The collected liquids were combined and lyophilized to obtain about 1 mg of product HYNIC-VQ (with a yield of about 35%) with a purity greater than 95%. ESI-MS mass spectrum analysis result: m / z =976.19 ([M+H] + ), C 43 H 71 N 14 O 10 S] + The t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com