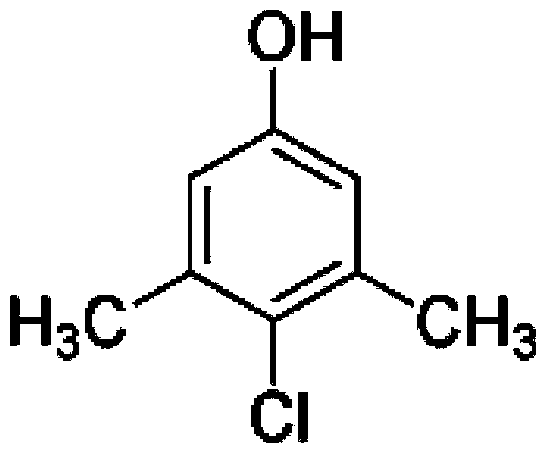
Preparing method for 4-chlorine-3,5-xylenol
A technology of dimethylphenol and divalent copper, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems such as the reduction of PCMX yield, and achieve the effect of high total conversion rate and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
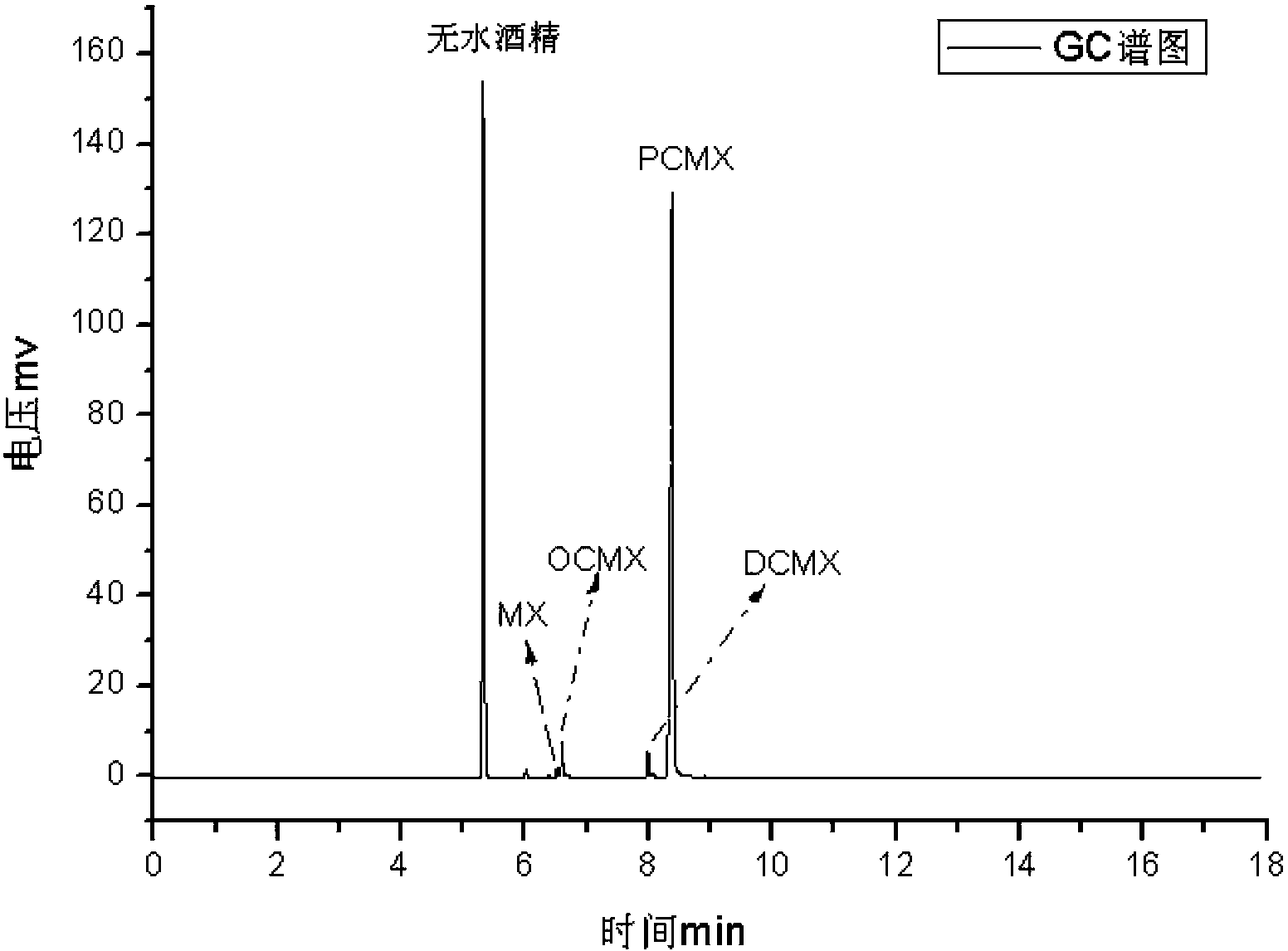
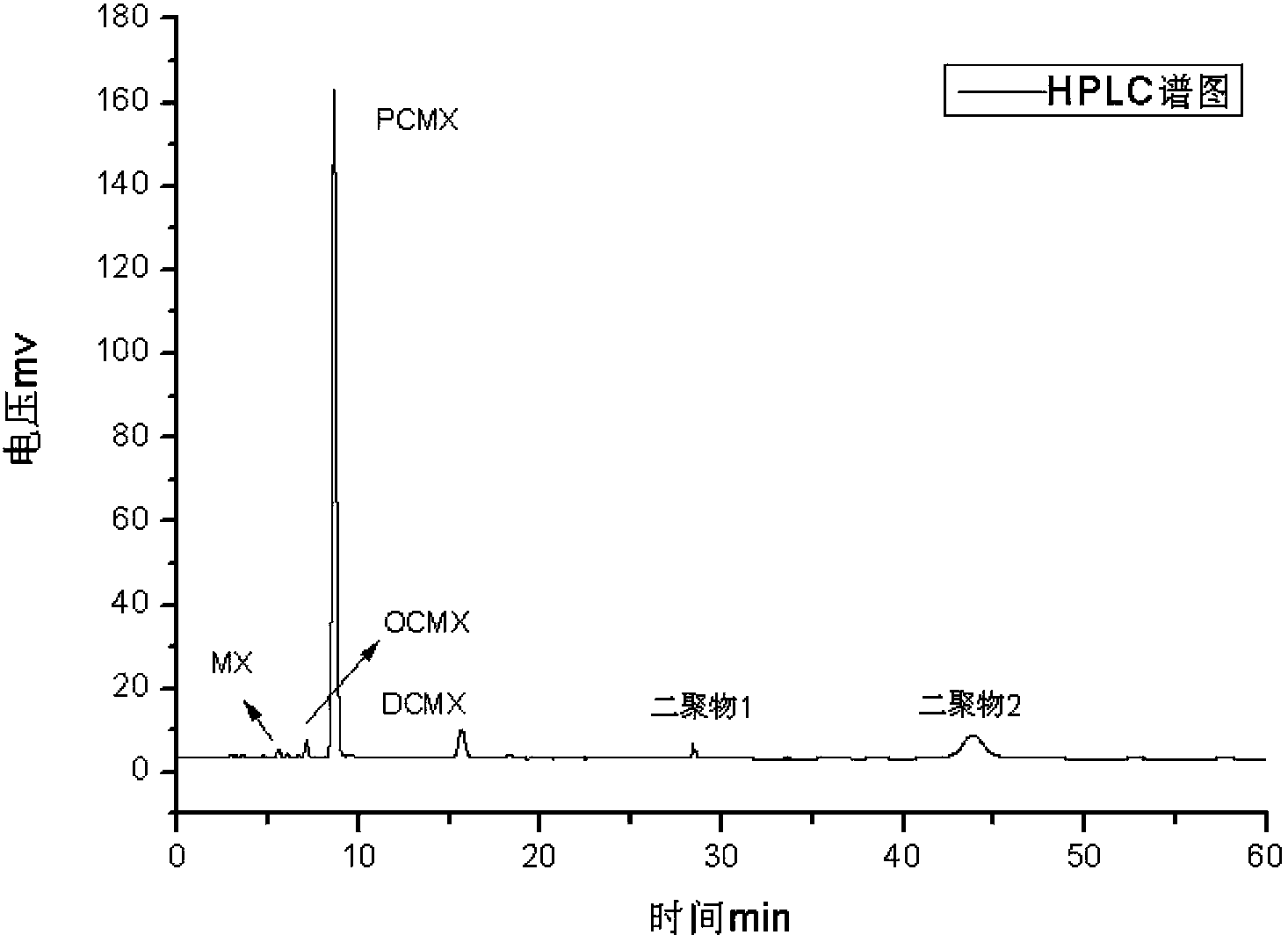
[0032] Add 1222g (10mol) 3,5-dimethylphenol, 244.4g copper chloride dihydrate, and 861g concentrated hydrochloric acid (HCl8.5mol) respectively in the 5000m1 three-necked reaction flask; then install a condenser, magnetic stirring, thermometer, and Oxygen tube. The reactant was heated to 90°C, and oxygen gas was passed in at a constant speed while stirring. The reaction temperature is controlled at 90°C, the reaction pressure is normal pressure, and the conversion rate is monitored by sampling in the middle of the reaction. The reaction ends when the reaction conversion rate reaches about 85%. After the reaction, the upper organic phase was taken to detect the content by HPLC (see Table 1). The upper organic phase was rectified, and the former fraction (3,5-dimethylphenol) (content 98%) was recovered, and the content of the obtained product was detected by HPLC (see Table 2).
Embodiment 2
[0034]Add 1222g (10mol) 3,5-dimethylphenol, 244.4g copper chloride dihydrate (CuCl 2 1.43mol), and 861g concentrated hydrochloric acid (HCl8.5mol); then install a condenser, magnetic stirring, thermometer, and oxygen tube. The reactant was heated to 95°C, and oxygen gas was passed in at a constant speed while stirring. The reaction temperature is controlled at 95°C, the reaction pressure is normal pressure, the conversion rate is monitored by sampling during the reaction, and the conversion rate is controlled at about 85%. After the reaction, the upper organic phase was taken to detect the content by HPLC (see Table 1). The upper organic phase was rectified, and the unreacted front fraction 3,5-dimethylphenol (content 98.1%) was recovered, recrystallized and washed to obtain the target product, and the content was detected by HPLC (see Table 2).
Embodiment 3
[0036] Add 1222g (10mol) 3,5-dimethylphenol, 244.4g copper chloride dihydrate (CuCl 2 1.43mol), and 861g concentrated hydrochloric acid (HCl8.5mol); then install a condenser, magnetic stirring, thermometer, and oxygen tube. The reactant was heated to 98°C, and oxygen gas was passed in at a constant speed while stirring. The reaction temperature is controlled at 98°C, the reaction pressure is normal pressure, the conversion rate is monitored by sampling during the reaction, and the conversion rate is controlled at about 85%. After the reaction, the upper organic phase was taken to detect the content by HPLC (see Table 1). The upper organic phase was rectified to recover the unreacted 3,5-dimethylphenol (content 98.3%), recrystallized and washed to obtain the target product, and the content was detected by HPLC (see Table 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com