Photoresist monomer, photoresist and their preparation method, color filter
A photoresist and monomer technology, which is applied in optical filters, optical mechanical equipment, sulfonate preparation, etc., can solve problems such as high hardness, light leakage, and large slope angle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
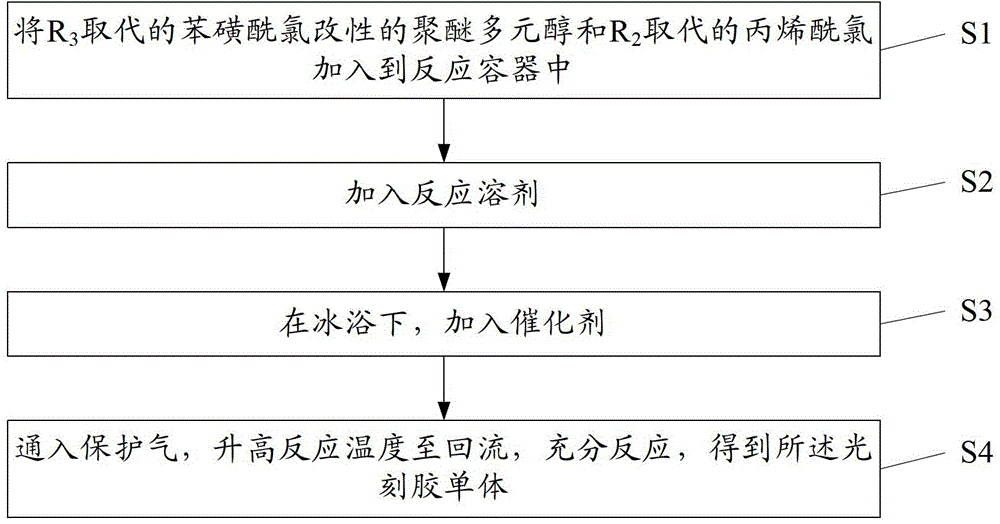
[0091] Corresponding to the aforementioned photoresist monomer, the embodiment of the present invention also provides a method for preparing the photoresist monomer. by figure 1 As shown, specifically, the method includes the following steps:
[0092] Step S1: Set R 3 Substituted benzenesulfonyl chloride modified polyether polyol and R 2 The substituted acryloyl chloride is added to the reaction vessel.
[0093] In this step, weigh an appropriate amount of reactant R 3 Substituted benzenesulfonyl chloride modified polyether polyol, R 2 The substituted acryloyl chloride is added to the reaction vessel. Preferably, the R used in this step 3 Substituted benzenesulfonyl chloride modified polyether polyol, R 2 The weight part of the substituted acryloyl chloride is, R 3 Substituted benzenesulfonyl chloride modified polyether polyol: 1 part; R 2 Substituted acryloyl chloride: 2.3 to 6.1 parts.
[0094] Step S2: Add reaction solvent.
[0095] In this step, weigh an appropriate amount of rea...
Embodiment 1
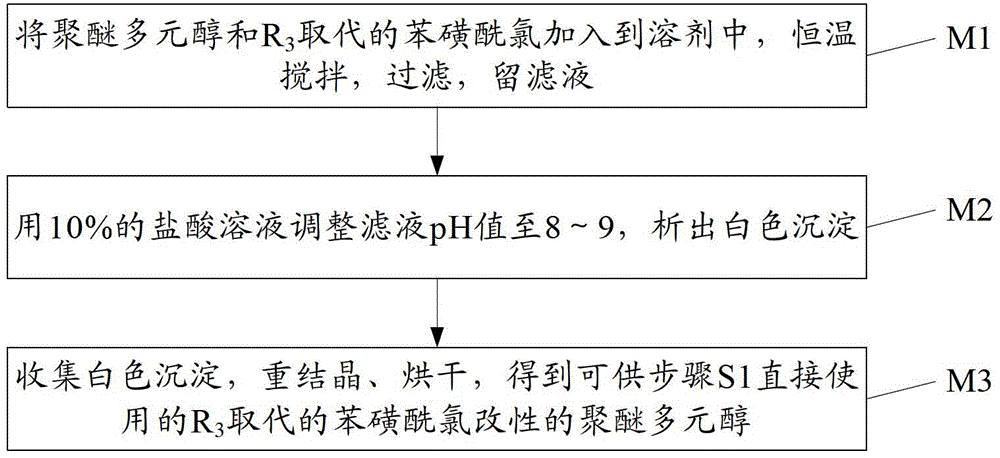
[0116] Preparation of methyl substituted benzenesulfonyl chloride modified polyether triol:
[0117] 2 parts of polyether triol and 0.9 part of p-toluenesulfonyl chloride were dissolved in 100 parts of acetonitrile solvent, and stirred at room temperature for 2 hours. The insoluble matter was removed by filtration, and the pH of the filtrate was adjusted to 8.2 with a 10% hydrochloric acid solution, and a large amount of white precipitate was deposited. The precipitate was collected by filtration, recrystallized three times with water, and dried under vacuum at 50°C for 12 hours to obtain a methyl-substituted benzenesulfonyl chloride modified polyether triol.
[0118] Gel permeation chromatography was used to test methyl-substituted benzenesulfonyl chloride modified polyether triol, and its molecular weight was 8875.
[0119] Preparation of photoresist monomer A:
[0120] Add 1 part of methyl-substituted benzenesulfonyl chloride modified polyether triol and 2.3 parts of 2-methacryloy...
Embodiment 2
[0125] Preparation of polyether tetraol containing methyl substituted polyether chain modified by ethyl substituted benzenesulfonyl chloride:
[0126] 2 parts of polyether tetraol containing methyl substituted polyether chains and 0.8 part of p-ethylbenzenesulfonyl chloride are dissolved in 120 parts of acetonitrile solvent, and stirred for 2.5 hours at a constant temperature. The insoluble matter was removed by filtration, and the pH value of the solution was adjusted to 8.4 with 10% hydrochloric acid solution, and a large amount of white precipitate was deposited. The precipitate was collected by filtration, recrystallized three times with water, and dried under vacuum at 50°C for 12 hours to obtain an ethyl-substituted benzenesulfonyl chloride modified polyether tetraol containing methyl-substituted polyether chains.
[0127] Gel permeation chromatography was used to test an ethyl-substituted benzenesulfonyl chloride modified polyether tetraol containing methyl-substituted polye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com