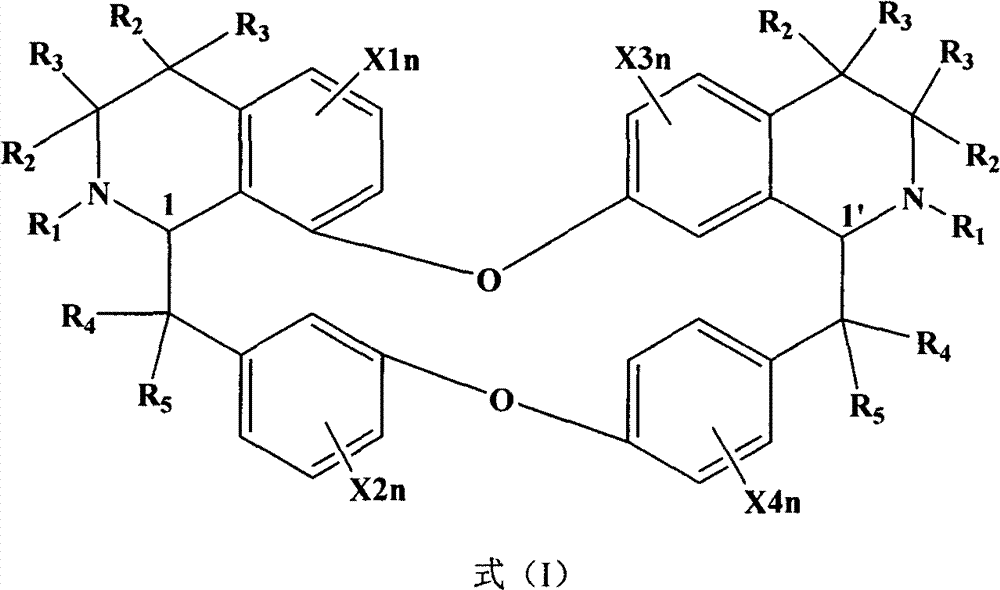
Novel use of bisbenzylisoquinoline compounds or pharmaceutically acceptable salts thereof in treating or improving depressive symptom
A technology of bisbenzylisoquinoline and compounds, applied in the field of new applications of bisbenzylisoquinoline compounds or their pharmaceutically acceptable salts in the treatment and improvement of depressive symptoms, capable of solving problems such as no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
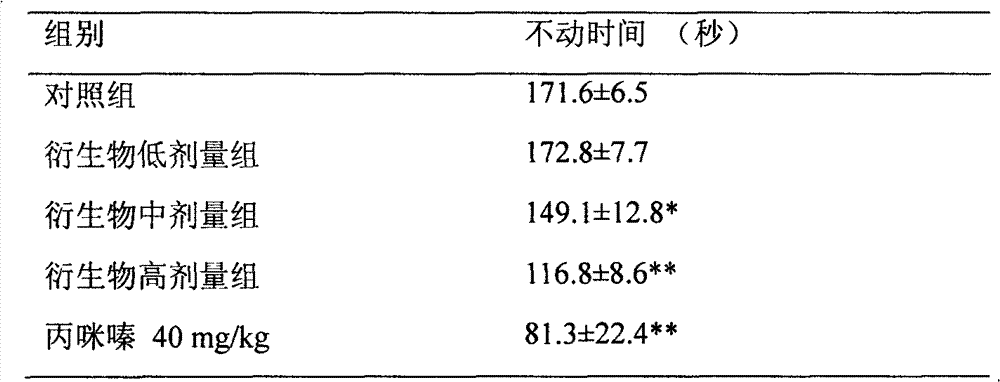
[0012] Experimental example 1, the effect of bisbenzylisoquinoline (BBI) derivatives represented by formula (I) on the immobility time of mice forced to swim.
[0013] The immobility of mice in the forced-swim model mirrors the animal's desperation behavior and mimics the depressive state in humans. Put the mice into a cylindrical glass jar with a height of 20 cm and a diameter of 10 cm, with a water depth of 15 cm and a water temperature of 25±1°C. The mice were pre-swimmed for 15 minutes before the experiment, then taken out, dried in a warm place, and then put back into the cage. Within 24 hours, the mice were administered intragastrically, and 60 minutes later, the mice were placed in the above-mentioned environment, and the cumulative immobility time of the mice in the last 5 minutes after swimming in the tank for 6 minutes was measured. The shortening of immobility time was used as an index to judge the antidepressant effect of drugs.
[0014] Experimental grouping: mi...
experiment example 2
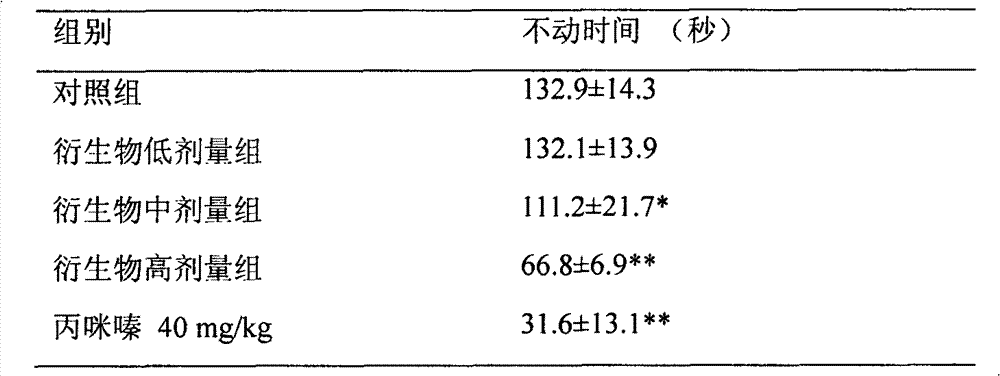
[0018] Experimental example 2, the effect of bisbenzylisoquinoline (BBI) derivatives represented by formula (I) on the tail suspension time of mice.
[0019] Same as Experiment 1, the immobility state of mice in the forced swimming model reflects the desperate behavior of animals, which can simulate the depressive state of humans. Mouse tail suspension test: 60 minutes after intragastric administration of mice, stick the mouse tail on a sling with glue strips at a distance of 2 cm from the tail tip of the mouse and hang it for 1 minute, and record the immobility time for 5 minutes.
[0020] Experimental grouping: mice were randomly divided into solvent control group (distilled water gavage), derivative (low: 15mg / kg; middle: 30mg / kg; high: 60mg / kg, gavage) group, and imipramine was a positive control ( 40mg / kg, gavage) group.
[0021] Table 2. Effect of derivatives on tail suspension time of rats (means±SEM, n=7).
[0022]
[0023] *P<0.05, **P<0.01 vs. control group (Stu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com