Cobalt complex conjugated microporous polymer catalyst, and preparation and application thereof
A technology of conjugated microporous and metallic cobalt, applied in the field of cyclic carbonate, can solve problems such as harsh conditions, and achieve the effects of increasing solubility, improving reaction yield and shortening reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1), the synthesis method of Salen-Co: dissolve 0.75mmol Salen in 10ml dry anhydrous toluene, add the methanol solution of cobalt acetate (1mmolCo(OAc) 2 dissolved in 10ml of methanol), and refluxed at 80°C for 5h to obtain the desired Salen-Co compound;
[0037] 2), the synthetic method of Salen-Co-OAc: dissolve 0.65mmol Salen-Co in 6ml toluene and 18mlCH 2 Cl 2 In the mixed solvent, inject 6.5mmol of CH3 COOH, under the protection of argon, stirred at 25°C for 5h to obtain the desired Salen-Co-OAc compound; figure 2 shown;
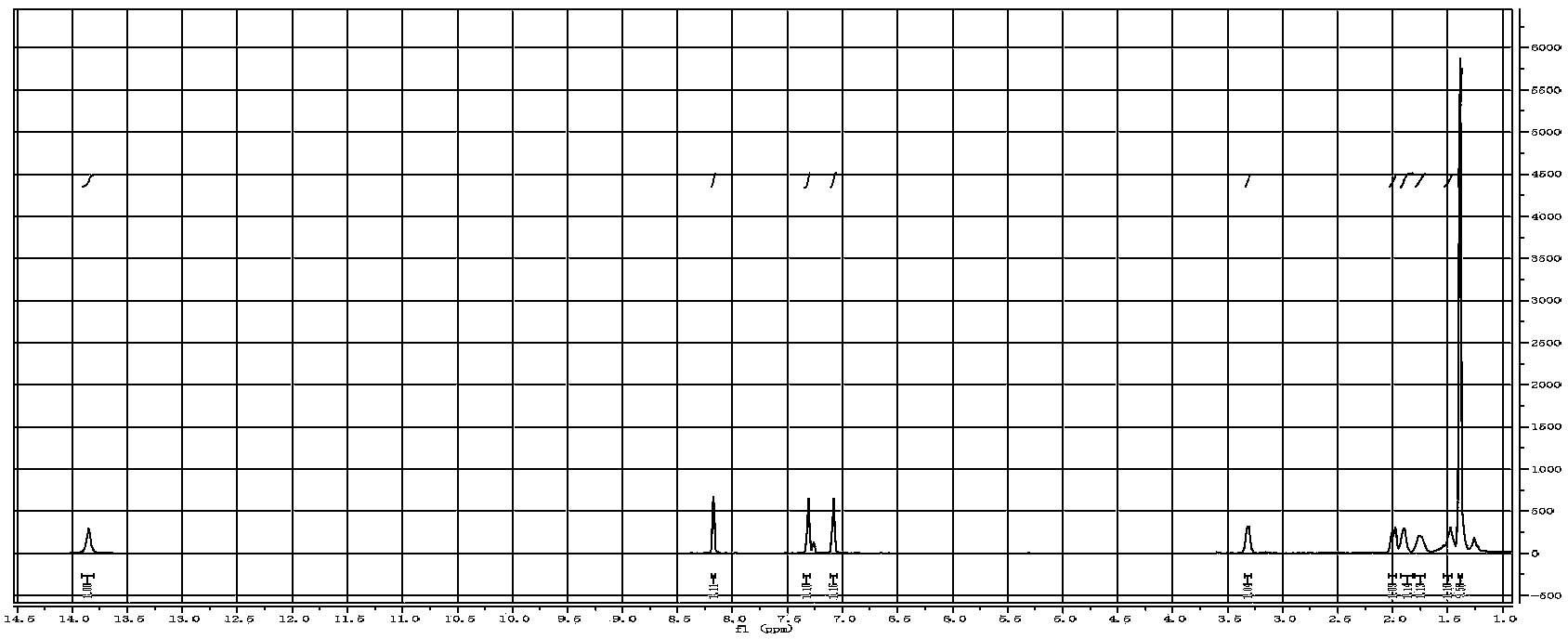
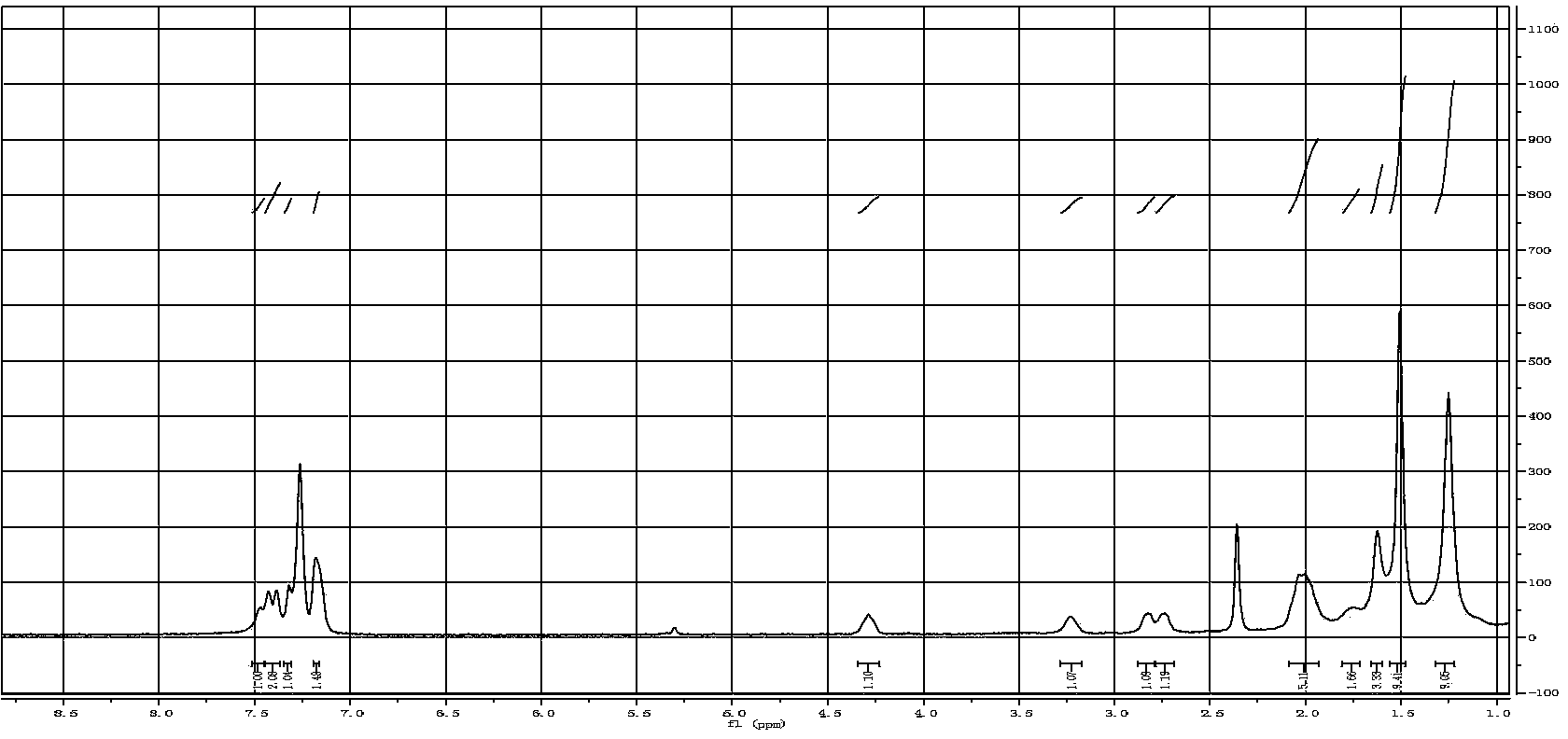
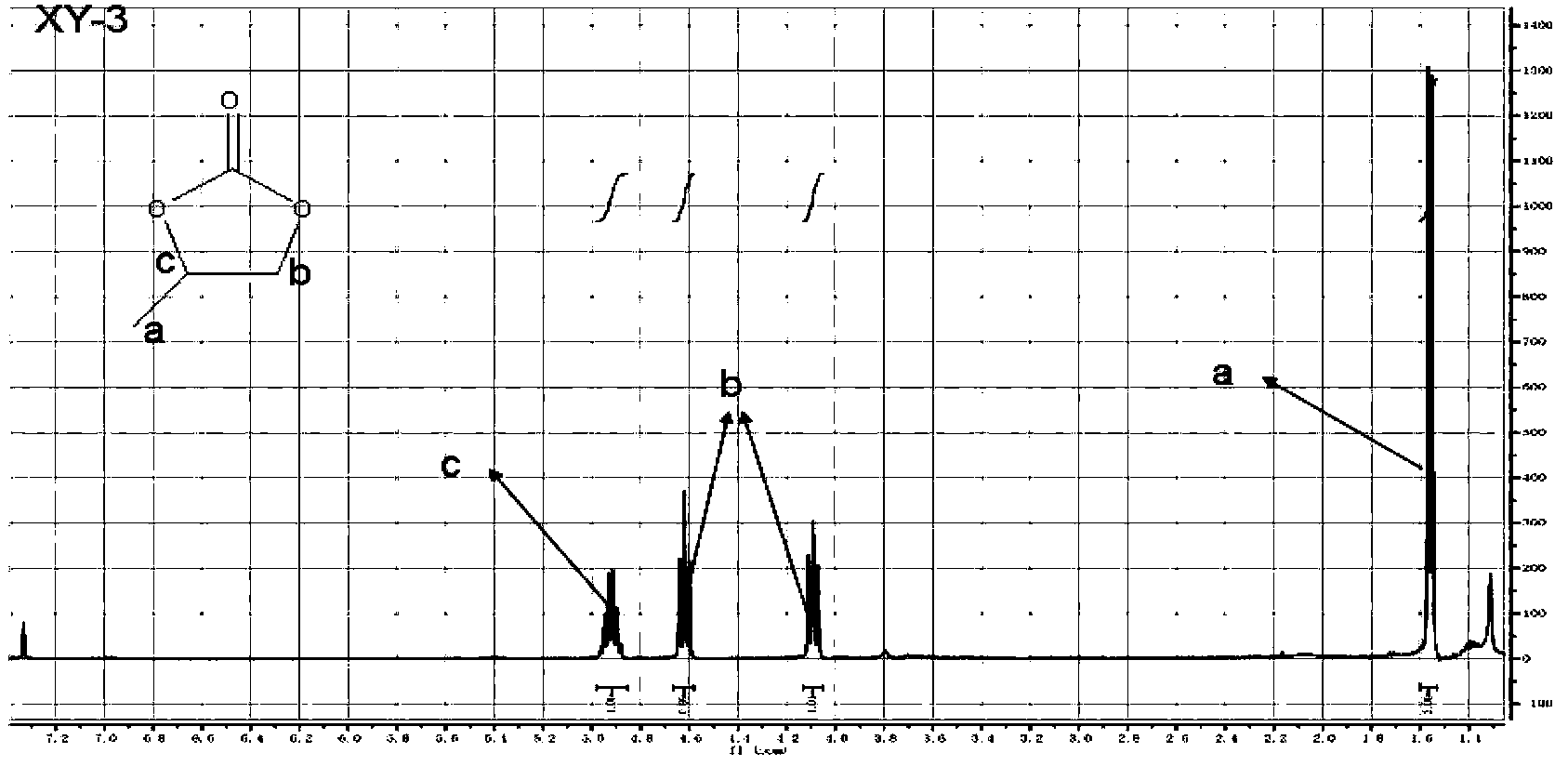
[0038] 3), the synthetic method of CMP-1: 0.45mmol Salen-Co-OAc, 1.35mmol 1,3,5-triethynylbenzene, 40mg CuI, 80mg tetrakis(triphenylphosphopalladium), use 15ml toluene and 5ml three Ethylamine was used as a solvent, refluxed at 85°C for 72h, and post-treated to obtain the desired CMP[Salen-Co-OAc] compound; the NMR of the compound was as follows Figure 5 shown;
[0039] 4), catalytic CO 2 Reaction with alkylene oxides:
[0040] ①, 100mg CM...
Embodiment 2
[0044] 1), the synthesis method of Salen-Co: dissolve 0.6mmol Salen in 10ml dry anhydrous toluene, add the methanol solution of cobalt acetate (1mmolCo(OAc) 2 dissolved in 10ml of methanol), and refluxed at 80°C for 5h to obtain the desired Salen-Co compound;
[0045] 2), the synthetic method of Salen-Co-OAc: 0.5mmol Salen-Co is dissolved in 5ml toluene and 15mlCH 2 Cl 2 In the mixed solvent, add 5.0mmol of CH by syringe 3 COOH, under the protection of argon, stirred at 25°C for 5h to obtain the desired Salen-Co-OAc compound; figure 2 shown;
[0046] 3), the synthetic method of CMP-1: 0.6mmol Salen-Co-OAc, 2.4mmol 1,3,5-triethynylbenzene, 60mg CuI, 100mg tetrakis(triphenylphosphopalladium), use 16ml toluene and 6ml tris Ethylamine was used as a solvent, refluxed at 85°C for 72h, and post-treated to obtain the desired CMP[Salen-Co-OAc] compound; the NMR of the compound was as follows Figure 5 shown;
[0047] 4), catalytic CO 2 Reaction with alkylene oxides:
[0048] ①...
Embodiment 3
[0052] 1), the synthesis method of Salen-Co: dissolve 0.5mmol Salen in 8ml dry anhydrous toluene, add the methanol solution of cobalt acetate (1mmolCo(OAc) 2 Dissolved in 8ml of methanol), refluxed at 80°C for 5h to obtain the desired Salen-Co compound;
[0053] 2), the synthetic method of Salen-Co-OAc: dissolve 0.65mmol Salen-Co in 5ml toluene and 15mlCH 2 Cl 2 In the mixed solvent, add 9.0mmol of CH by syringe 3 COOH, under the protection of argon, stirred at 25°C for 6h to obtain the desired Salen-Co-OAc compound; figure 2 shown;
[0054] 3), the synthetic method of CMP-1: 0.6mmol Salen-Co-OAc, 2.0mmol 1,3,5-triethynylbenzene, 50mg CuI, 100mg tetrakis(triphenylphosphopalladium), use 16ml toluene and 5ml tris Ethylamine was used as a solvent, refluxed at 85°C for 72h, and post-treated to obtain the desired CMP[Salen-Co-OAc] compound; the NMR of the compound was as follows Figure 5 shown;
[0055] 4), catalytic CO 2 Reaction with alkylene oxides:
[0056] ①, 100mg C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com