Microneedle devices and methods
A microneedle and medical device technology, applied in the direction of microneedles, needles, pharmaceutical formulations, etc., can solve the problem of expensive system manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0174] Preparations containing lidocaine and clonidine
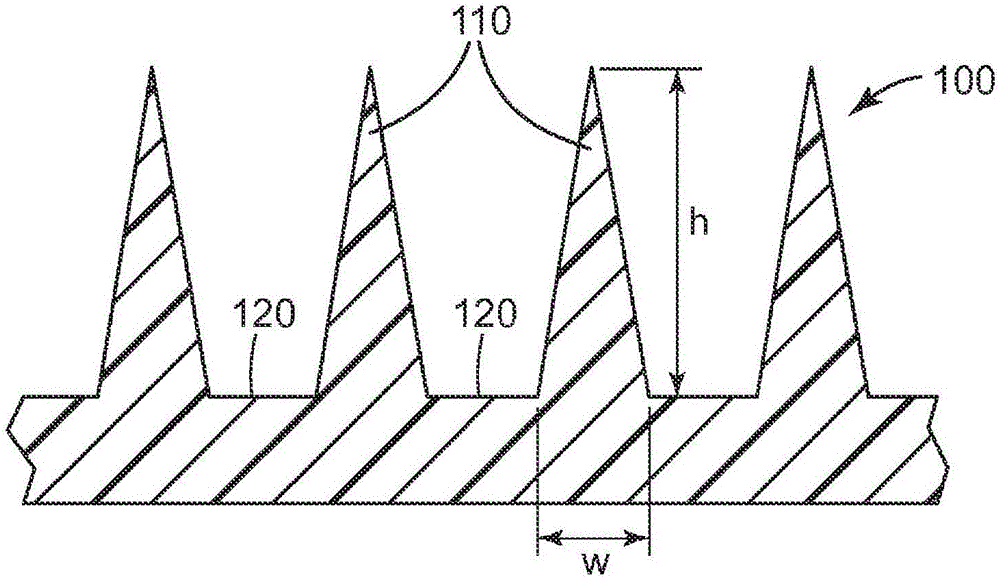
[0175] The microneedle array uses a surface area of about 1.27 cm 2 Class VI medical grade liquid crystal polymer (LCP) ( MT1300, injection molded from Ticona Plastics, Auburn Hills, Michigan, USA (3M Company, St. Paul, MN, USA). Each microneedle array is characterized by 316 quadrangular pyramidal microneedles arranged in an octagonal pattern, where the height of the microneedles is nominally 500 μm, the aspect ratio is about 3:1, and the tips between adjacent microneedles reach The tip distance is nominally 550 microns.
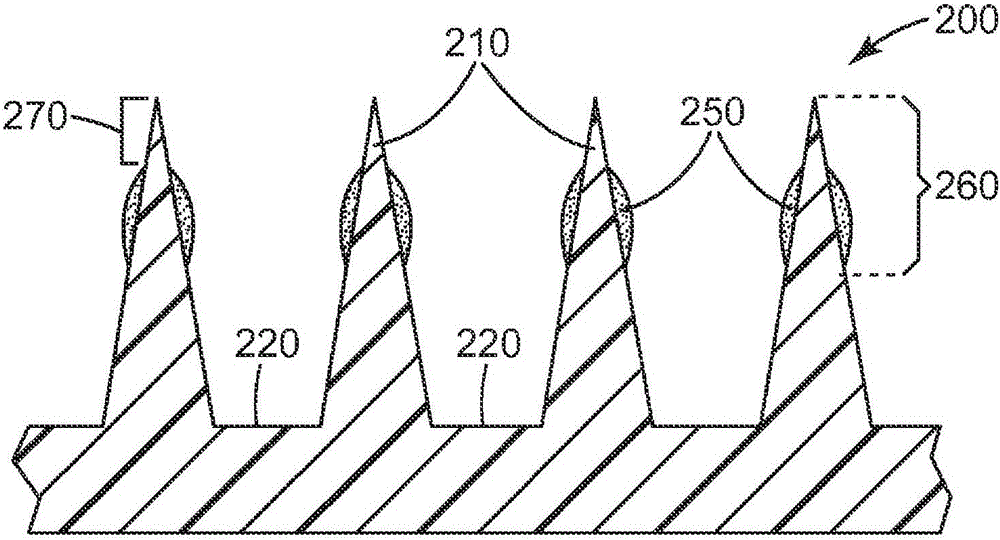
[0176] Using the dip-coating method, a mixture of 30% dextran (Pharmacosmos, Holbaek, Denmark), 30% lidocaine hydrochloride (Sigma-Aldrich, St. Sigma, St. Louis, MO)) and 0.3% clonidine hydrochloride (Spectrum Chemical & Laboratory Products, New Brunswick, NJ, USA (Spectrum Chemical & Laboratory Products, New Brunswick, NJ)), lidocaine was coated onto the microneedle array. Prior to coating, ...
example 2
[0187] Preparations containing lidocaine and epinephrine
[0188] The microneedle array uses a surface area of about 1.27 cm 2 Class VI medical grade liquid crystal polymer (LCP) ( MT1300, injection molded from Ticona Plastics, Auburn Hills, Michigan, USA (3M Company, St. Paul, MN, USA). Each microneedle array is characterized by 316 quadrangular pyramidal microneedles arranged in an octagonal pattern, where the height of the microneedles is nominally 500 μm, the aspect ratio is about 3:1, and the tips between adjacent microneedles reach The tip distance is nominally 550 microns.
[0189] A mixture of 30% dextran (Pharmacosmos, Holbaek, Denmark), 30% lidocaine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was used by dip coating. Sigma, St.Louis, MO)) and 0.03% epinephrine tartrate (Sigma, St.Louis, MO, USA, Sigma, St.Louis, MO) to apply lidocaine to the microneedle array superior. Prior to coating, microneedle arrays were cleaned with 70% isopropanol (BDH, West ...
example 3
[0200] Preparations containing prilocaine and clonidine
[0201] The microneedle array uses a surface area of about 1.27 cm 2 Class VI medical grade liquid crystal polymer (LCP) ( MT1300, injection molded from Ticona Plastics, Auburn Hills, Michigan, USA (3M Company, St. Paul, MN, USA). Each microneedle array is characterized by 316 quadrangular pyramidal microneedles arranged in an octagonal pattern, where the height of the microneedles is nominally 500 μm, the aspect ratio is about 3:1, and the tips between adjacent microneedles reach The tip distance is nominally 550 microns.
[0202] A mixture of 30% dextran (Pharmacosmos, Holbaek, Denmark), 15% prilocaine hydrochloride (Spectrum Chemical & Laboratory Products, New Brunswick, NJ, USA) was used by dip coating. Spectrum Chemical & Laboratory Products, New Brunswick, NJ)) and 0.15% clonidine hydrochloride (Spectrum Chemical & Laboratory Products of New Brunswick, New Jersey, USA (Spectrum Chemical & Laboratory Product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com