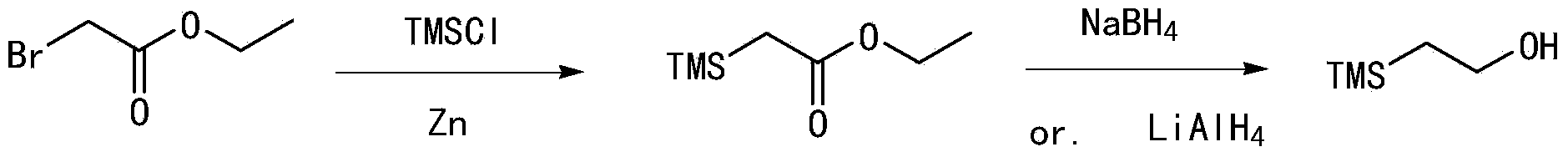
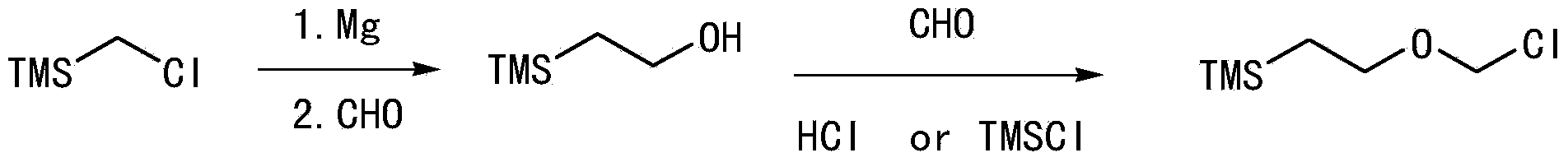
Synthetic method of 2-(trimethylsilyl)-ethoxymethyl chloride
A technology of ethoxymethyl chloride and trimethylsilyl group, applied in the field of 2-ethoxymethyl chloride synthesis, can solve the problems of low cost, cost restriction, high price and the like, and achieves cost reduction, energy saving and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0030] Example 2 According to the steps in Example 1, the type of the second step trimethylsilyl coupling reaction is adopted format exchange method:
[0031]
[0032] After exchange with the isopropyl format, the target product can also be obtained according to the above method, and the yield is 85%.
Embodiment 3
[0033] Example 3 According to the steps in Example 1, the type of the second step trimethylsilyl coupling reaction adopts the butyllithium method:
[0034]
[0035] Using butyllithium to react at low temperature, the target product can also be obtained according to the above method, and the yield is 55%.
Embodiment 4
[0036] Example 4 According to the steps in Example 1, the type of the second step trimethylsilyl coupling reaction described herein adopts a strong base method:
[0037]
[0038] Reaction with a strong base can also obtain the target product according to the above method, and the yield is 20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com