B cell vaccine based on Hepal-6 hepatoma cell autophagosome-DRibbles and preparation method of B cell vaccine
A technology of hepatoma cells and autophagosomes, which can be applied to tumors/cancer cells, animal cells, vertebrate cells, etc., can solve the problem of insufficient strength, and achieve the effect of reducing harm and improving immune response ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
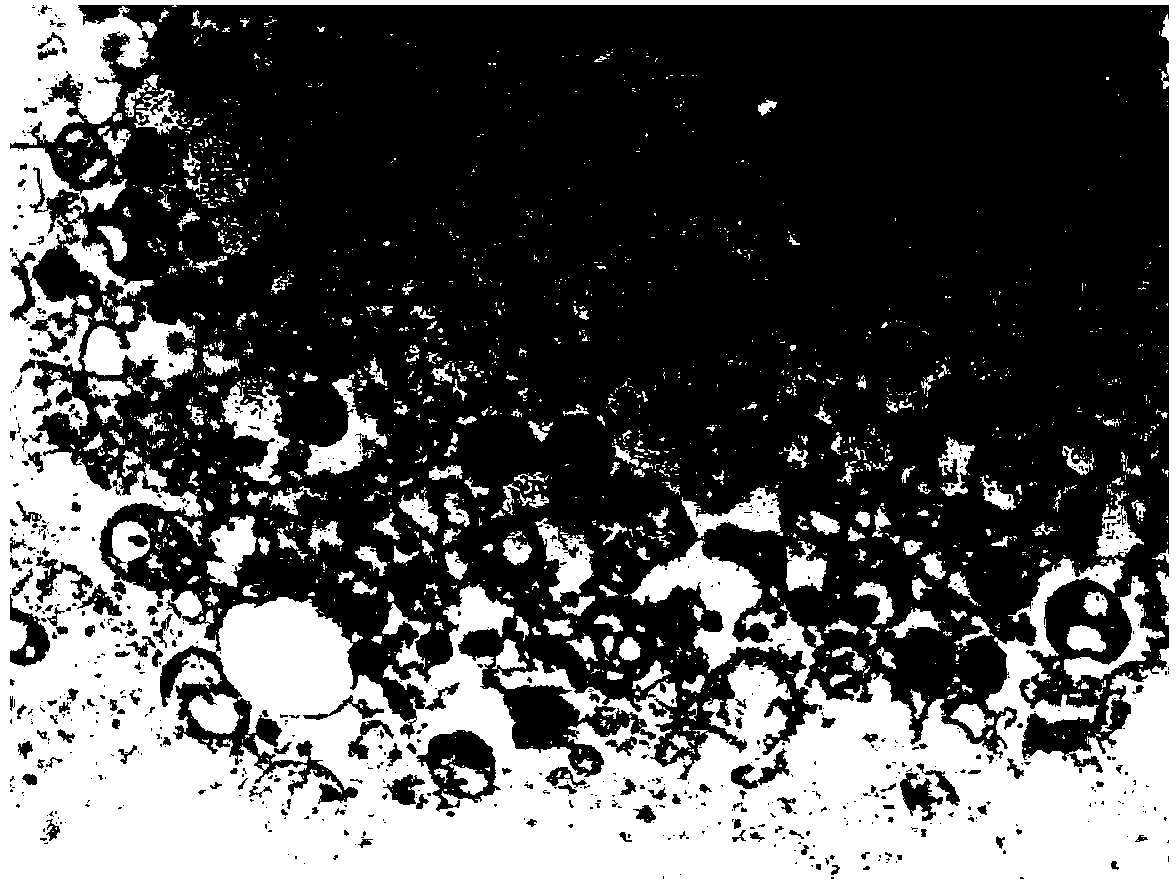
[0058] Example 1: Electron Microscopic Observation of the Morphology of Autophagosomes-DRibbles in Hepa1-6 Liver Cancer Cells
[0059] The extracted Hepa1-6 liver cancer cell autophagosome-DRibbles was centrifuged at 12000rpm at a high speed to precipitate and compress tightly, discard the supernatant, and Hepa1-6 liver cancer cell autophagosome-DRibbles was precipitated with 2.5% (v / v) pentadiene Aldehyde was fixed, processed in the electron microscope room, and the morphology of autophagosomes-DRibbles of Hepa1-6 liver cancer cells was observed on the microscope.
[0060] Such as figure 1 Shown: Under the electron microscope, the autophagosomes of Hepa1-6 liver cancer cells-DRibbles are small bodies with a double-layer membrane structure, with an average diameter of about 300nm-1μm (pointed by the arrow), which proves that the autophagosomes of liver cancer cells are effectively recruited .
Embodiment 2
[0061] Example 2: Hepa1-6 liver cancer cell autophagosomes-DRibbles induce B cell proliferation and secretion of IgM antibodies in vitro
[0062] 1. Hepa1-6 liver cancer cell autophagosomes-DRibbles induce B cell proliferation and secretion of IgM antibodies in vitro
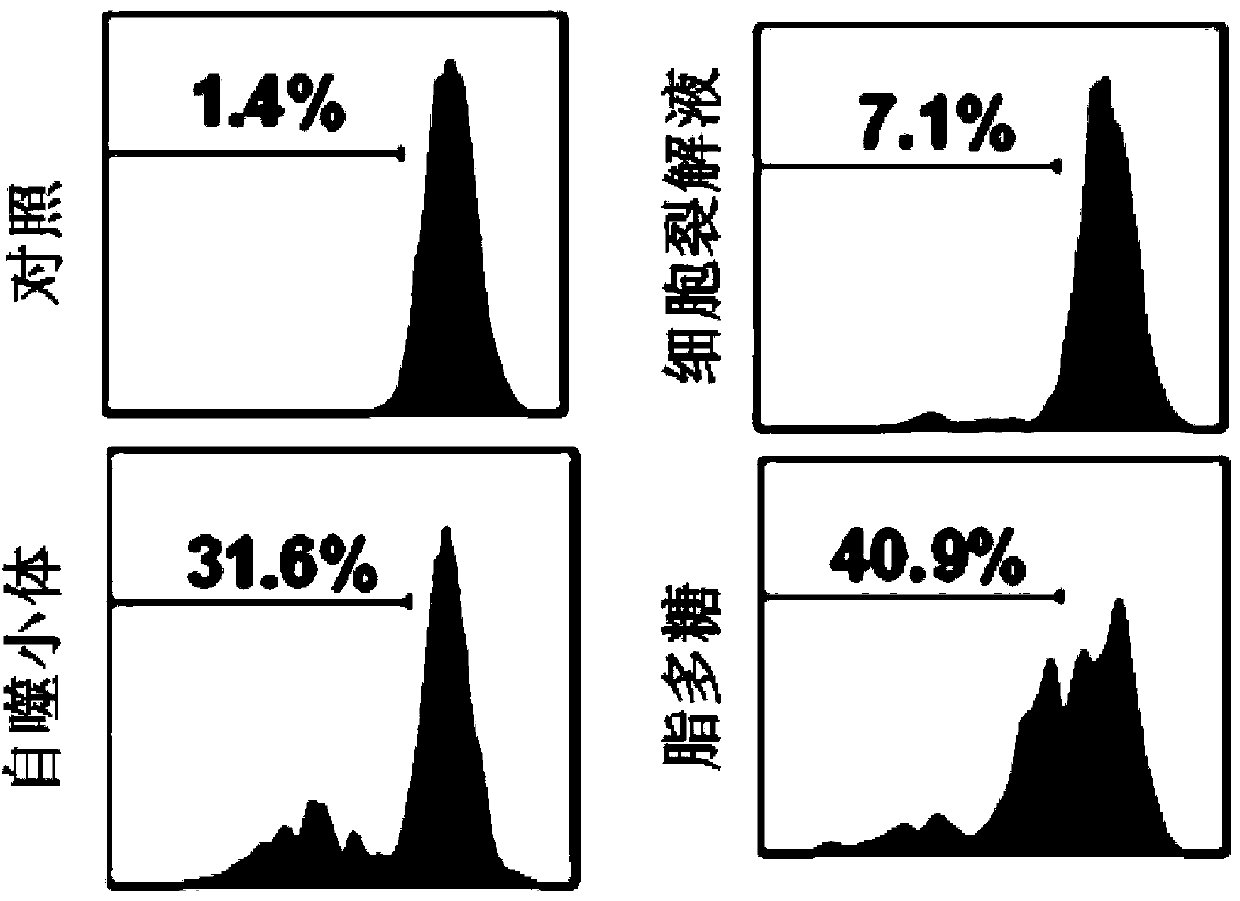
[0063] ①Hepa1-6 liver cancer cell autophagosomes-DRibbles stimulate B cell proliferation experiment
[0064] Take C57 / BL6 mouse splenocytes, use CD43 negative selection magnetic beads to separate mouse spleen B cells, and mark B cells with 5uM CFSE. After that, CFSE-labeled B cells were stimulated with 10ug / ml Hepa1-6 liver cancer cell autophagosome-DRibbles, Hep1-6 cell lysate and 10ug / ml LPS respectively. After 5 days, B cells were collected by centrifugation at 1000rpm, and CFSE was identified by flow cytometry + The division and proportion of B cells.
[0065] Such as figure 2 As shown, the B cells stimulated by the autophagosome-DRibbles of Hepa1-6 liver cancer cells can promote 31.6% of B cells to div...
Embodiment 3
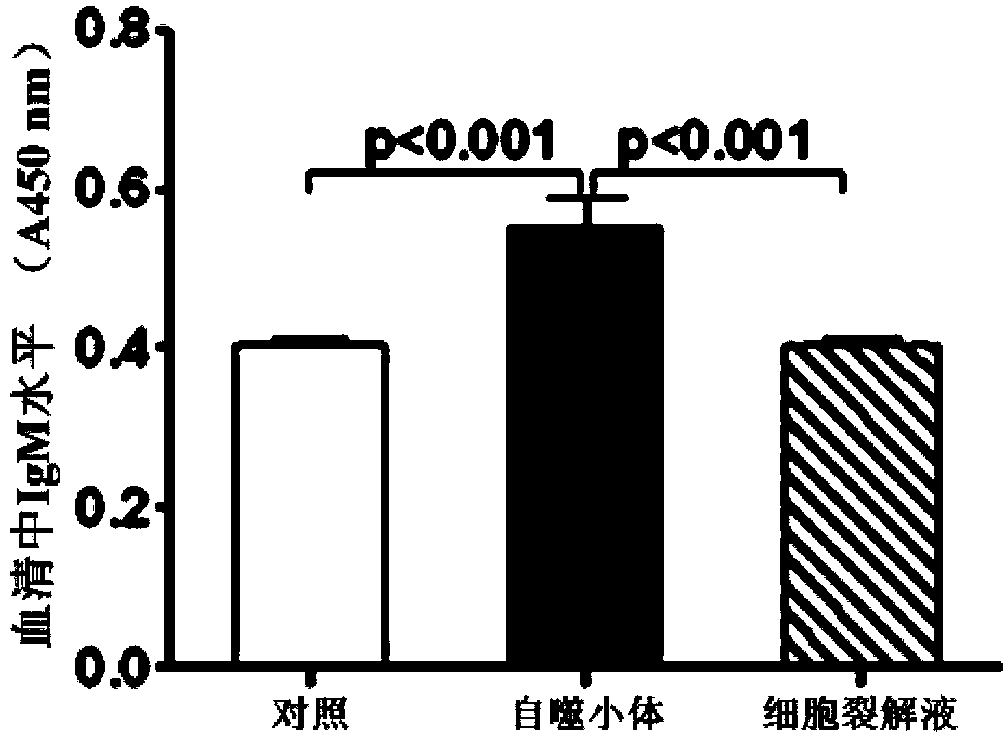
[0069] Example 3: Hepa1-6 liver cancer cell autophagosomes-DRibbles induce B cells to produce specific antibodies in vivo
[0070] Inject C57 / BL6 mice (30ug total protein / mouse) via tail vein with Hepa1-6 liver cancer cell autophagosomes-DRibbles on the 1st, 2nd, and 3rd day, collect blood from orbit on the 7th day, separate serum (frozen at -20°C) save).
[0071] 1. Detection of IgM and IgG in serum (ELISA method)
[0072] ① Coat the 96-well plate with anti-mouse IgM or IgG (1:10000) and leave overnight at 4°C.
[0073] ② Wash the plate 3 times with PBST (phosphate buffered saline PBS containing 0.5% Tween), 3 minutes each time. Then block with 2% goat serum blocking solution, 37°C, 2h.
[0074] ③ Wash the plate 3 times with PBST. Serum samples were diluted (1:500, 1:1000, 1:2000...), added to a sealed 96-well plate, and incubated at 37°C for 1 hour.
[0075] ④ Wash the plate 5 times with PBST, add HRP-antimouse-IgM or IgG (1:10000), and incubate at 37°C for 1h.
[0076...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com