Carvedilol-asccharin amorphous compound
An amorphous, carvedilol technology, applied in the field of medicine, can solve the problems of obvious first-pass effect, only 25% absolute bioavailability, insoluble in water, etc.
Inactive Publication Date: 2013-12-25
CHINA PHARM UNIV
View PDF4 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Carvedilol is a class II drug in the biopharmaceutical classification system, which is insoluble in water and has a significant first-pass effect, making its absolute bioavailability only 25%-35%
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
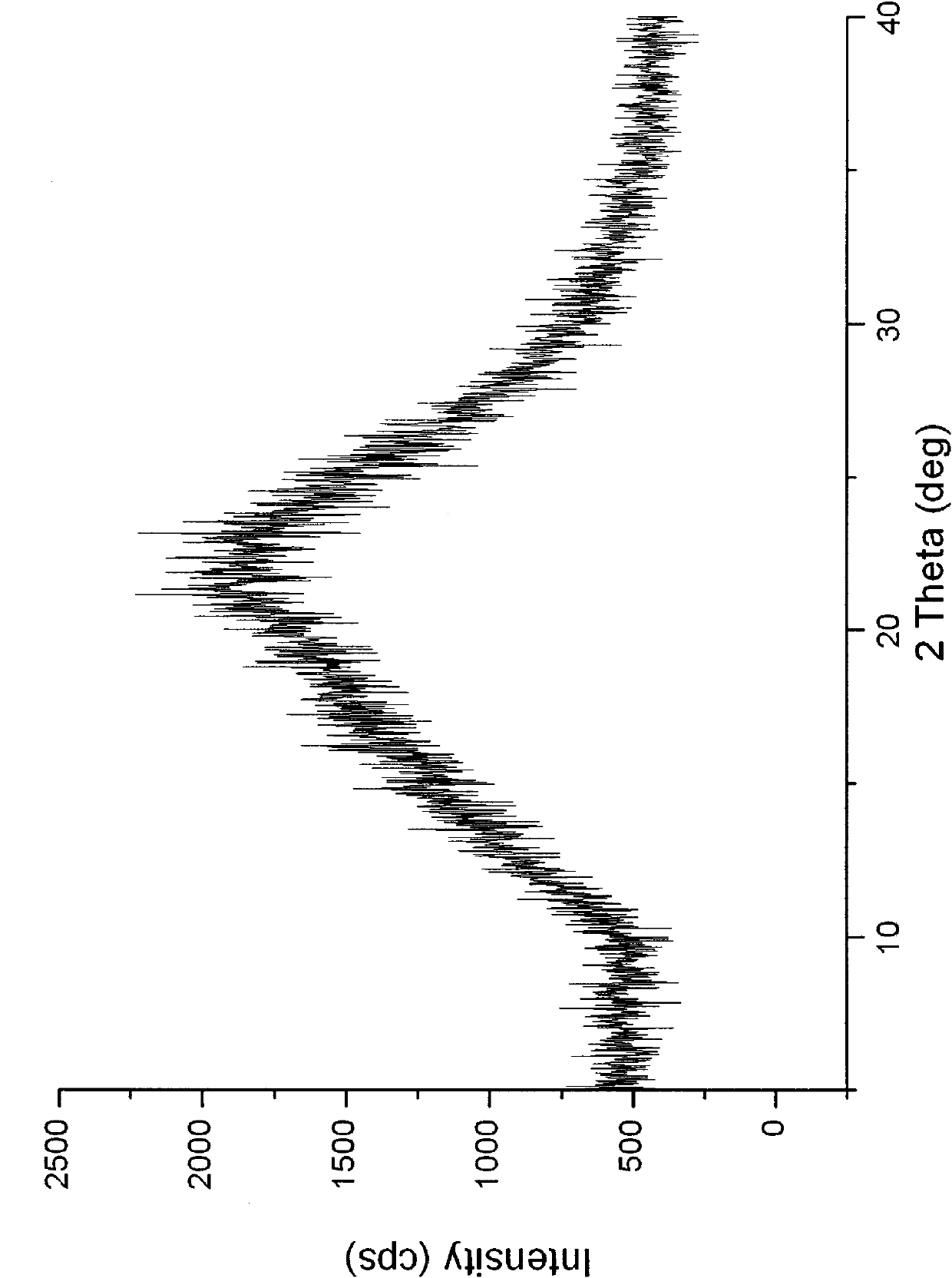
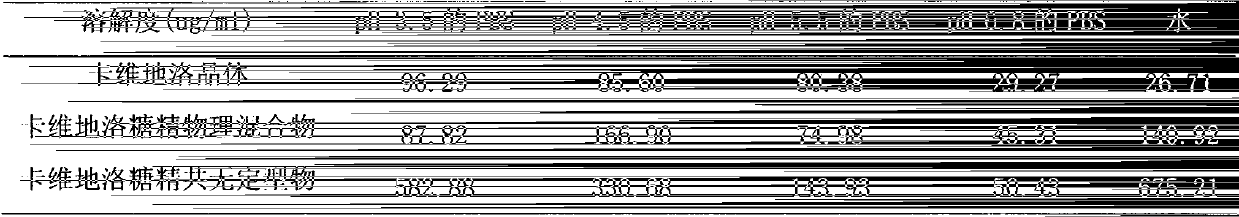
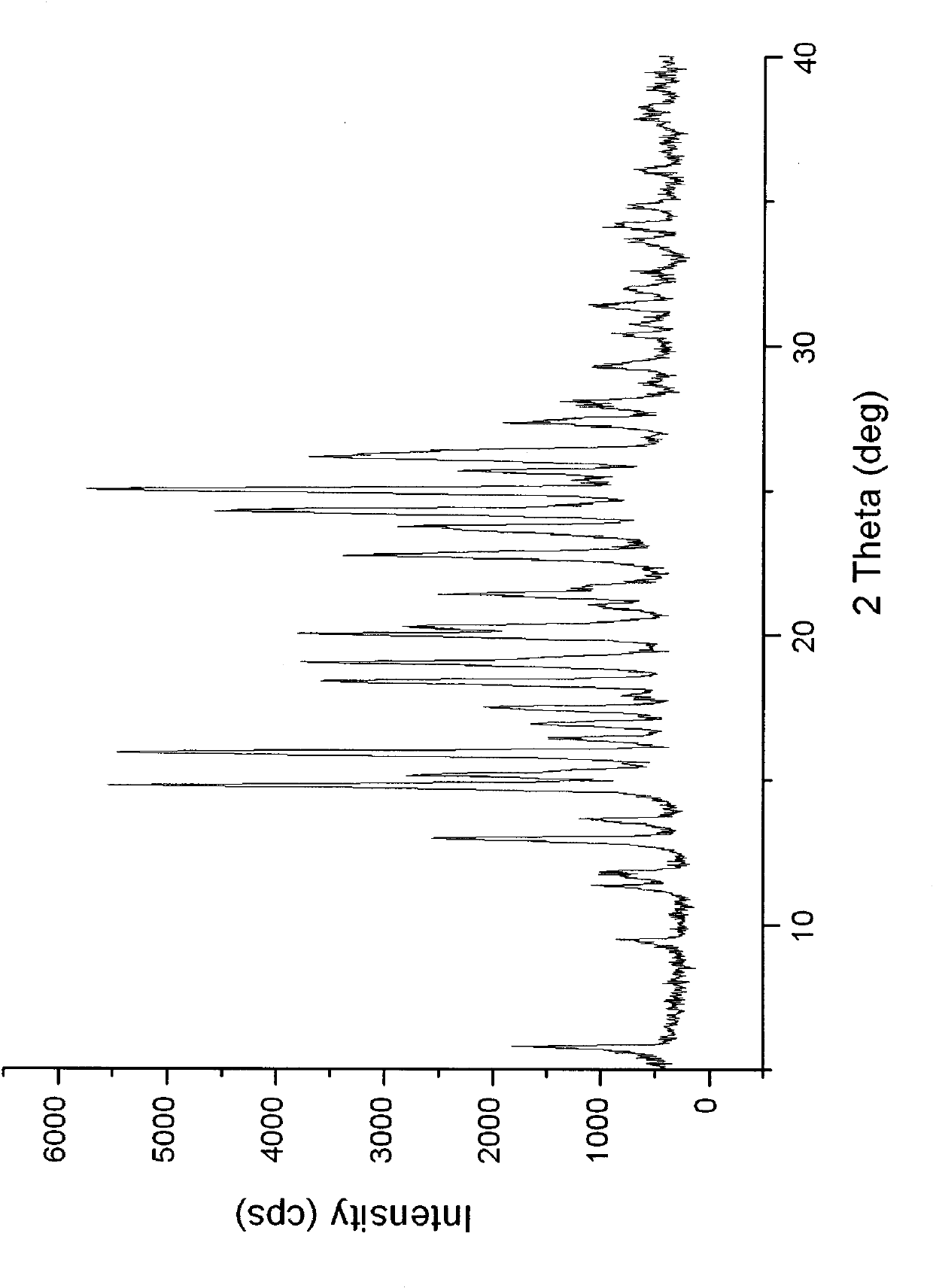
The invention relates to a carvedilol-asccharin amorphous compound. The carvedilol-asccharin amorphous compound is obtained by combination of carvedilol and asccharin. Based on Cu-K alpha radiation, an X-ray powder diffraction spectrum represented by a degree of 2 theta does not comprise a sharp diffraction peak. The powder X-ray diffraction spectrum of the carvedilol-asccharin amorphous compound is different from those of carvedilol crystals and a physical mixture of carvedilol crystals and asccharin crystals and thus the carvedilol-asccharin amorphous compound is a novel amorphous compound completely different from carvedilol crystals and the physical mixture of carvedilol crystals and asccharin crystals. The carvedilol-asccharin amorphous compound can obviously improve solubility of carvedilol in various mediums.
Description
Technical field The invention belongs to the technical field of medicine, and specifically relates to a carvedilol saccharin co-amorphous substance formed by combining carvedilol and saccharin at a molar ratio of 1:1 and a preparation method thereof. Background technique Carvedilol, whose chemical name is 1-carbazole-4-oxy-3-[2-(2-methoxyphenoxy)ethylamino]-2-propanol, is a new type of vasodilator . Clinically, it can be used to treat essential hypertension and cardiac insufficiency. Carvedilol has both α1 and non-selective β-receptor blocking effects within the therapeutic dose range, and has no intrinsic sympathomimetic activity. This product blocks post-synaptic membrane α receptors, thereby dilating blood vessels and reducing peripheral vascular resistance; blocking β receptors, inhibits kidney secretion of renin, blocks the renin-angiotensin-aldosterone system, and reduces blood pressure effect. Carvedilol lowers blood pressure quickly and can maintain the lowering ef...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D209/88C07D275/06
Inventor 郝君吴敏张建军高缘
Owner CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com