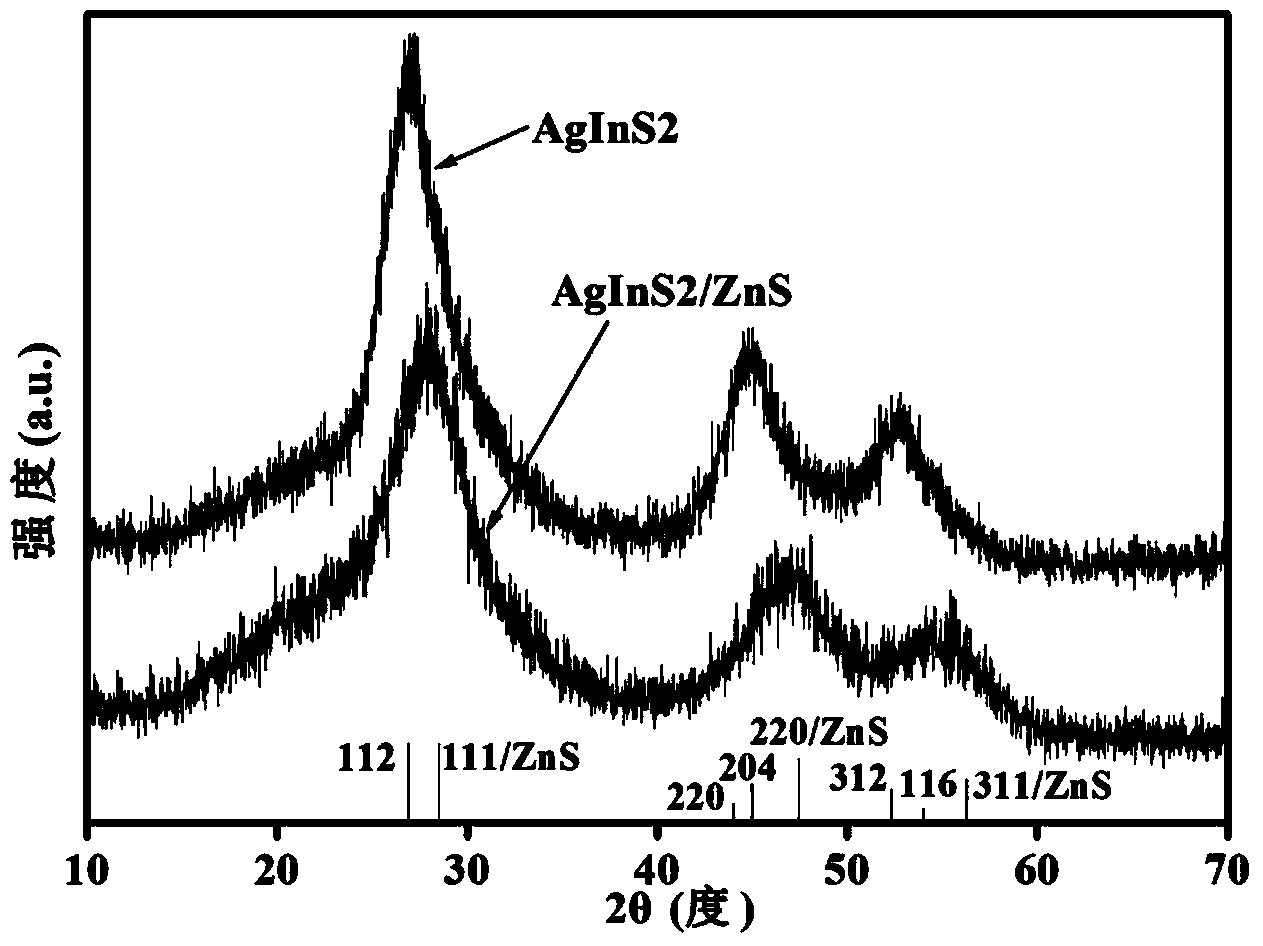
Fluorescent AgInS2 and AgInS2/ZnS nano-crystal prepared by microwave-assisted method
A technology of nanocrystals and nanocrystals, applied in the field of fluorescent AgInS2 and AgInS2/ZnS nanocrystals, which can solve the problems of preparing complex precursors, reducing quantum yield and stability, and being difficult to directly apply to the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1. Fluorescent AgInS 2 and AgInS 2 / ZnS nanocrystal preparation
[0020] AgInS 2 Nanocrystals were synthesized by microwave irradiation with 0.06 ml of 0.1M AgNO 3 Aqueous solution, 0.4 mL 0.1M In(NO 3 ) 3 The aqueous solution and 1.6 ml of 0.1 M glutathione aqueous solution were sequentially added to 18.4 mL of deionized water, and the pH was adjusted to 8.5 with 1 M NaOH. During this process, the solution changed from cloudy to clear. Then add 0.8 ml of 0.05M Na 2 S aqueous solution. Then the above solution was transferred to a microwave reactor with a volume of 80 ml, and reacted with microwave radiation for 5 minutes, the temperature was set at 100° C., and the power was 200 W. Cool to 50°C to obtain fluorescent AgInS 2 Nanocrystalline.
[0021] AgInS2 / Preparation of ZnS nanocrystals. The above-prepared AgInS 2 nanocrystals, add 0.8 ml of 0.1M Zn(AC) 2 aqueous solution and 0.8 mL of 0.05M Na 2 S aqueous solution, continue microwave radiation ...
Embodiment 2
[0023] Example 2. Fluorescent AgInS 2 and AgInS 2 / ZnS nanocrystal preparation
[0024] AgInS 2 Nanocrystals were synthesized by microwave irradiation with 0.08 ml of 0.1M AgNO 3 Aqueous solution, 0.4 mL 0.1M In(NO 3 ) 3 The aqueous solution and 1.6 ml of 0.1 M glutathione aqueous solution were sequentially added to 18.4 ml of deionized water to adjust the pH to 8.5. During this process, the solution changed from cloudy to clear. Then add 0.8 ml of 0.05M Na 2 S aqueous solution. Then the above solution was transferred to a microwave reactor with a volume of 80 ml, and reacted with microwave radiation for 5 minutes, the temperature was set at 100° C., and the power was 200 W. Cool to 50°C to obtain fluorescent AgInS 2 Nanocrystalline.
[0025] AgInS 2 / Preparation of ZnS nanocrystals. The above-prepared AgInS 2 nanocrystals, add 0.8 ml of 0.1M Zn(AC) 2 aqueous solution and 0.8 mL of 0.05M Na 2 S aqueous solution, continue microwave radiation reaction for 5 minut...
Embodiment 3
[0027] Example 3. Fluorescent AgInS 2 and AgInS 2 / ZnS nanocrystal preparation
[0028] AgInS 2 Nanocrystals were synthesized by microwave irradiation with 0.1 ml of 0.1M AgNO 3 Aqueous solution, 0.4 mL 0.1M In(NO 3 ) 3 The aqueous solution and 1.6 ml of 0.1 M glutathione aqueous solution were sequentially added to 18.4 ml of deionized aqueous solution to adjust the pH to 8.5. During this process, the solution changed from cloudy to clear. Then add 0.8 ml of 0.05M Na 2 S aqueous solution. Then the above solution was transferred to a microwave reactor with a volume of 80 ml, and reacted with microwave radiation for 5 minutes, the temperature was set at 100° C., and the power was 200 W. Cool to 50°C to obtain fluorescent AgInS 2 Nanocrystalline.
[0029] AgInS 2 / Preparation of ZnS nanocrystals. The above-prepared AgInS 2 nanocrystals, add 0.8 ml of 0.1M Zn(AC) 2 aqueous solution and 0.8 mL of 0.05M Na 2 S aqueous solution, continue microwave radiation reaction f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com