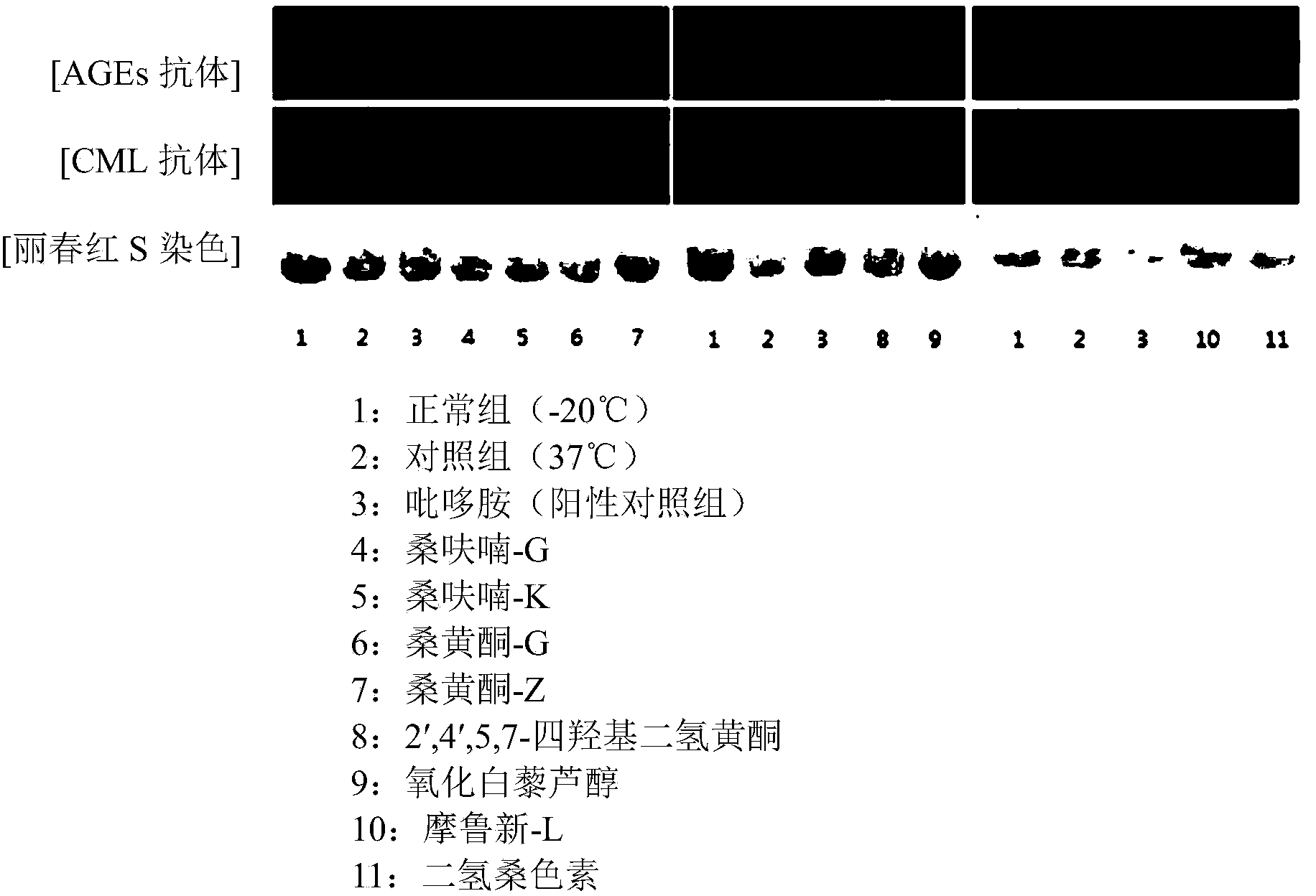
Use of compounds isolated from morus bark
A technology of mulberry white peel and separation, which is applied in the directions of active ingredients of hydroxy compounds, active ingredients of heterocyclic compounds, and medical preparations containing active ingredients, etc., and can solve problems such as unknown effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation and analysis of Moran furan G in Cortex Morus alba
[0066] 1-1: Morus alba methanol extract
[0067] Dried Morus alba (3kg) was extracted with 10L of methanol and refluxed repeatedly for 3 times, each time for 4 hours, filtered and then concentrated under reduced pressure to obtain 150g of methanol extract.
[0068] 1-2: Preparation of organic solvent isolate from Cortex Morus alba extract
[0069] Dissolve and disperse the Morus Alba methanol extract (150 g) prepared in 1-1 in 6 liters of water, and extract with n-hexane (3 liters 3 times) and ethyl acetate (3 liters 3 times) successively to obtain n-hexane extraction Extract (50g) and ethyl acetate extract (50g). The ethyl acetate extract was subjected to silica gel column chromatography (silica gel column 500g), and dichloroethane (CH 2 Cl 2 )-methanol (70%:30%, 50%:50%, 30%:70%, 10%:90%, 0:100%) solvent system gradient elution, and 25 isolates (MAE-01~25 ).
[0070] 1-3: Preparation of active iso...
Embodiment 2
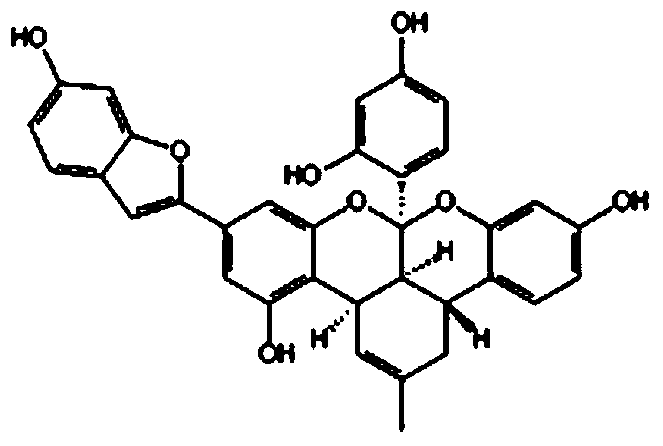
[0077] Preparation and analysis of Morus furan K in Cortex Morus alba
[0078] The ethyl acetate part MA.E-14 prepared in 1-2 in Example 1 was subjected to reversed-phase silica gel (RP-18) column chromatography (methanol:water=1:1→3:1 methanol, gradient washing Off), to obtain 20 isolates (MA.E-1401 ~ 1420). The obtained isolate MA.E-1417~1418 was subjected to reversed-phase silica gel chromatography (RP-18, methanol:water=1:1 (2000ml→2:1 (1000ml), separated and refined into 2 parts, and the chemical formula Moranfuran K (20㎎) in 2.
[0079] Moranfuran K: Pale yellow crystalline powder.
[0080] 1H-NMR (400MHz, DMSO-d6): 1.24 (3H, s, H-24″), 1.29 (3H, s, H-25″), 1.74 (3H, s, H-1″), 1.92 (1H , dd, J=11.4, 16.2Hz, H-6″), 2.68 (1H, brs, H-6″), 2.77 (1H, m, H-5″), 3.18 (1H, brs, H-3″ ), 3.23 (1H, dd, J=11.5Hz, H-4″), 5.67 (1H, d, J=9.9Hz, H-22″), 6.23 (1H, brs, H-17″), 6.24 ( 1H, d, J=8Hz, H-13″), 6.31 (1H, brd, J=4.4Hz, H-2″), 6.41 (1H, dd, J=2.4, 8.4Hz, H-19″), 6.57 (1H,...
Embodiment 3
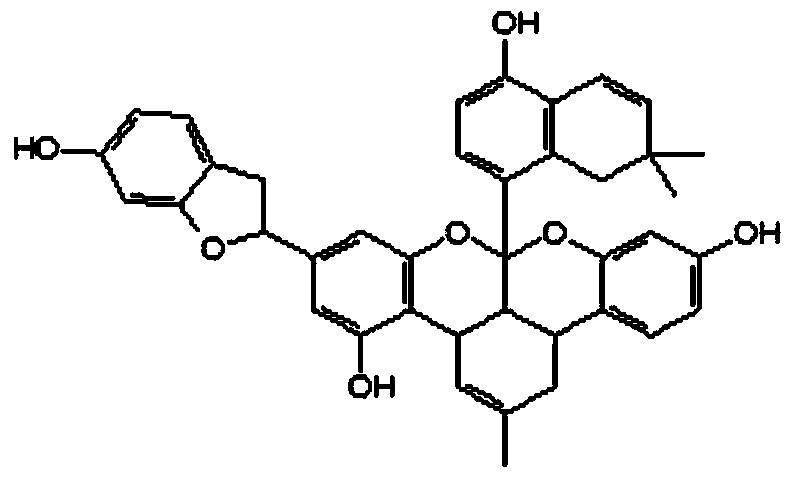
[0083] Preparation and Analysis of Morus alba G in Cortex Morus alba
[0084] The ethyl acetate part MA.E-17 prepared in 1-2 in Example 1 was subjected to reversed-phase silica gel (RP-18) column chromatography (methanol:water=1:1→2:1 methanol, gradient washing Off), to obtain 12 isolates (MA.E-1701 ~ 1712). The thus obtained isolate MA.E-1710 was subjected to preparative high performance liquid chromatography (HPLC, C18, 5μm, 19×150㎜i.d., 60% acetonitrile, 285nm, 5ml / min), after separation and purification, the phin flavonoid G (30㎎) in the chemical formula 3 was obtained.
[0085] Phin flavonoid G: Amorphous red powder.
[0086]1H-NMR (500MHz, acetone-d6): 7.34 (1H, H-14″), 7.27 (1H, H-6′), 6.76 (1H, H-20″), 6.65 (1H, H-3′) , 6.55 (1H, H-5′), 6.18 (1H, H-17″), 6.06 (1H, H-19″), 6.00 (1H, H-6), 5.96 (1H, H-11″), 5.93 (1H, H-13″), 5.20 (1H, H-2″), 5.16 (1H, H-12), 4.62 (1H, H-6″), 4.42 (1H, H-1″), 3.70 (1H, H-7″), 3.15 (1H, H-11), 3.14 (1H, H-11), 1.59 (3H, H-14), 1.51 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com