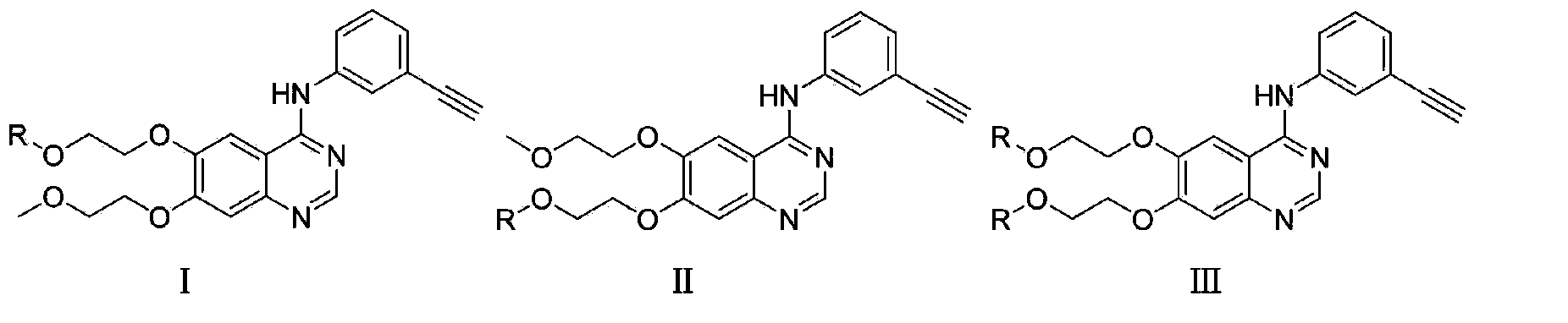
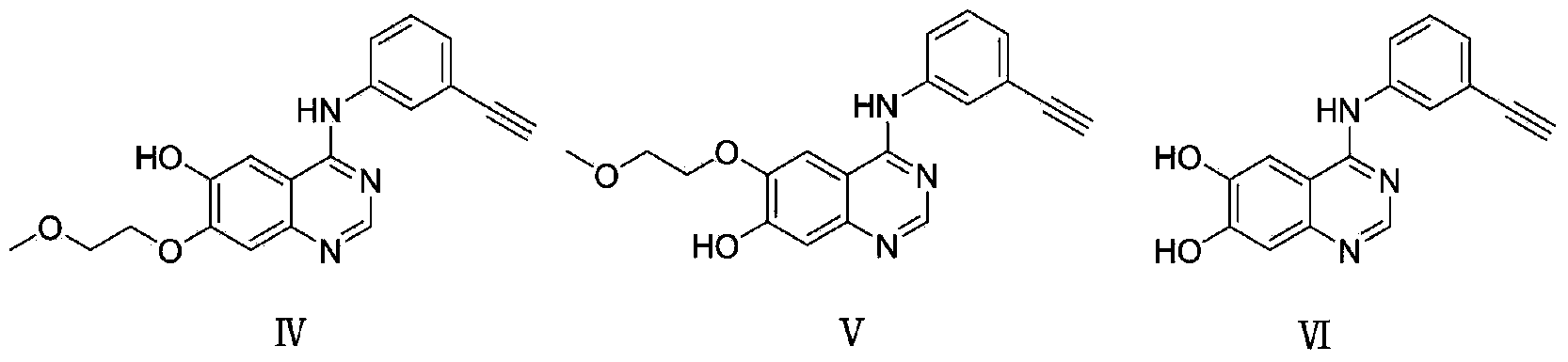
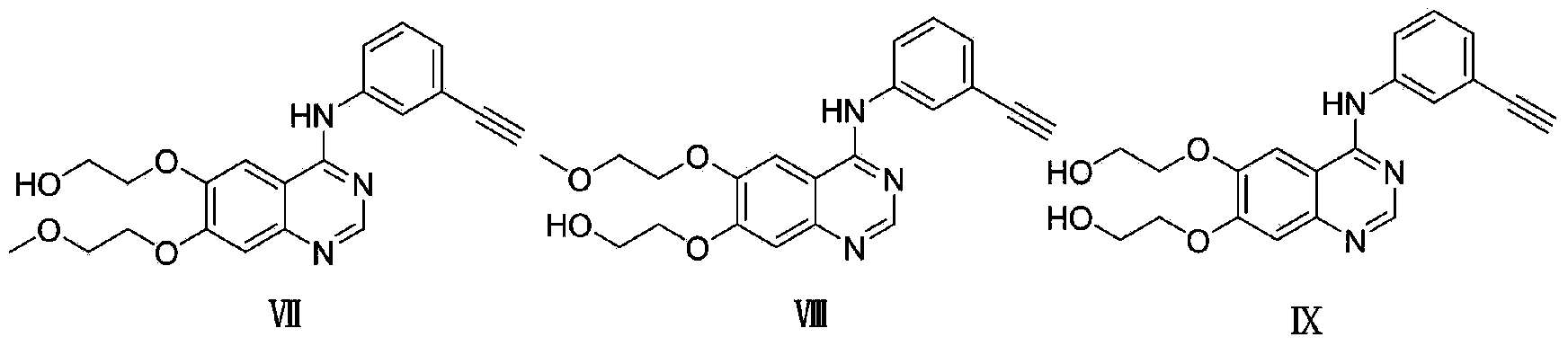
Coupling compound of NSAID anti-inflammatory pain killers and EGFR kinase inhibitor and synthetic method and application of coupling compound
A coupling compound, anti-inflammatory and analgesic technology, applied in the field of chemical medicine, can solve problems such as insensitivity and drug resistance of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 2-(4-(3-ethynylaniline)-7-(2-methoxyethoxy)quinazoline-6-oxyl)ethyl-2-(4-benzoylphenyl)propionic acid synthesis
[0031]
[0032] step 1
[0033] Synthesis of ethyl 3-hydroxy-4-(2-methoxyethoxy)benzoate
[0034]
[0035] Dissolve 10.9g of ethyl 3,4-dihydroxybenzoate in 40ml of dry DMF (dimethylformamide), slowly add 3.6g of NaH under ice-cooling, and slowly drop in 8.34g of 2-bromoethyl after 10min under the protection of argon A solution of methyl ether and 100 mg KI dissolved in 10 ml of dry DMF was added dropwise within 3 hours, during which the reaction temperature was maintained at 0°C. After the dropwise addition, the reaction temperature was naturally raised to room temperature, and the reaction was carried out overnight, followed by TLC until the end of the reaction. The reaction solution was diluted with water, extracted with ethyl acetate, washed successively with saturated sodium chloride solution, and dried over anhydrous sodium sulfate. The crude ...
Embodiment 2
[0077] 2-(4-(3-ethynylaniline)-7-(2-methoxyethoxy)quinazoline-6-oxyl)ethyl-2-(4-isobutylphenyl)propionic acid synthesis
[0078]
[0079] The synthesis method is as in Example 1.
[0080] 1 H NMR (400MHz, CDCl 3 )δ8.59(s,1H),7.83(s,1H),7.73(d,J=8.0Hz,1H),7.31-7.00(m,8H),4.52-4.46(m,1H),4.42-4.36 (m,1H),4.16-4.14(m,4H),3.73-3.68(m,3H),3.40(s,3H),3.06(s,1H),2.39(d,J=7.2Hz,2H), 1.81(m,1H),1.47(d,J=7.2Hz,3H),0.85(d,J=6.4Hz,6H); 13 C NMR (125MHz, CDCl 3 )δ174.5, 156.4, 154.1, 153.4, 148.6, 147.1, 140.5, 138.8, 137.3, 129.4, 129.2, 128.8, 127.6, 127.0, 125.1, 122.6, 122.4, 109.3, 106.5, 103.4, 77.3, 08, 83.3 ,69.2,66.5,62.3,59.2,44.9,29.6,22.3,18.5,13.9;
[0081] ESI + m / z568.3(M+H) + .
Embodiment 3
[0083] Synthesis of 2-(4-(3-ethynylaniline)-7-(2-methoxyethoxy)quinazoline-6-oxyl)ethyl-2-acetoxybenzoic acid
[0084]
[0085] The synthesis method is as in Example 1, wherein the acid chloride used in step 8 is a purchased product, not self-made.
[0086] 1 H NMR (400MHz, CDCl 3 )δ8.61(s,1H),8.08(br.,1H),7.96(d,J=8.0Hz,1H),7.83-7.80(m,1H),7.72(d,J=8.0Hz,1H) ,7.51(dt,J=1.6,8.0Hz,1H),7.29-7.16(m,5H),7.06(d,J=8.0Hz,1H),4.62(t,J=4.0Hz,2H),4.31( t,J=4.0Hz,2H),4.12(t,J=4.0Hz,2H),3.70(t,J=4.4Hz,2H),3.34(s,3H),3.09(s,1H),2.31( s,3H); 13 CNMR (125MHz, CDCl 3 )δ169.8, 164.0, 156.3, 153.9, 153.4, 150.6, 148.6, 133.9, 131.7, 129.9, 128.8, 127.5, 125.9, 123.6, 122.5, 122.3, 119.1, 117.4, 109.4, 107.7, 783.7, 103.3 ,76.7,70.6,68.9,66.5,62.8,20.8.
[0087] ESI + m / z542.0(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com