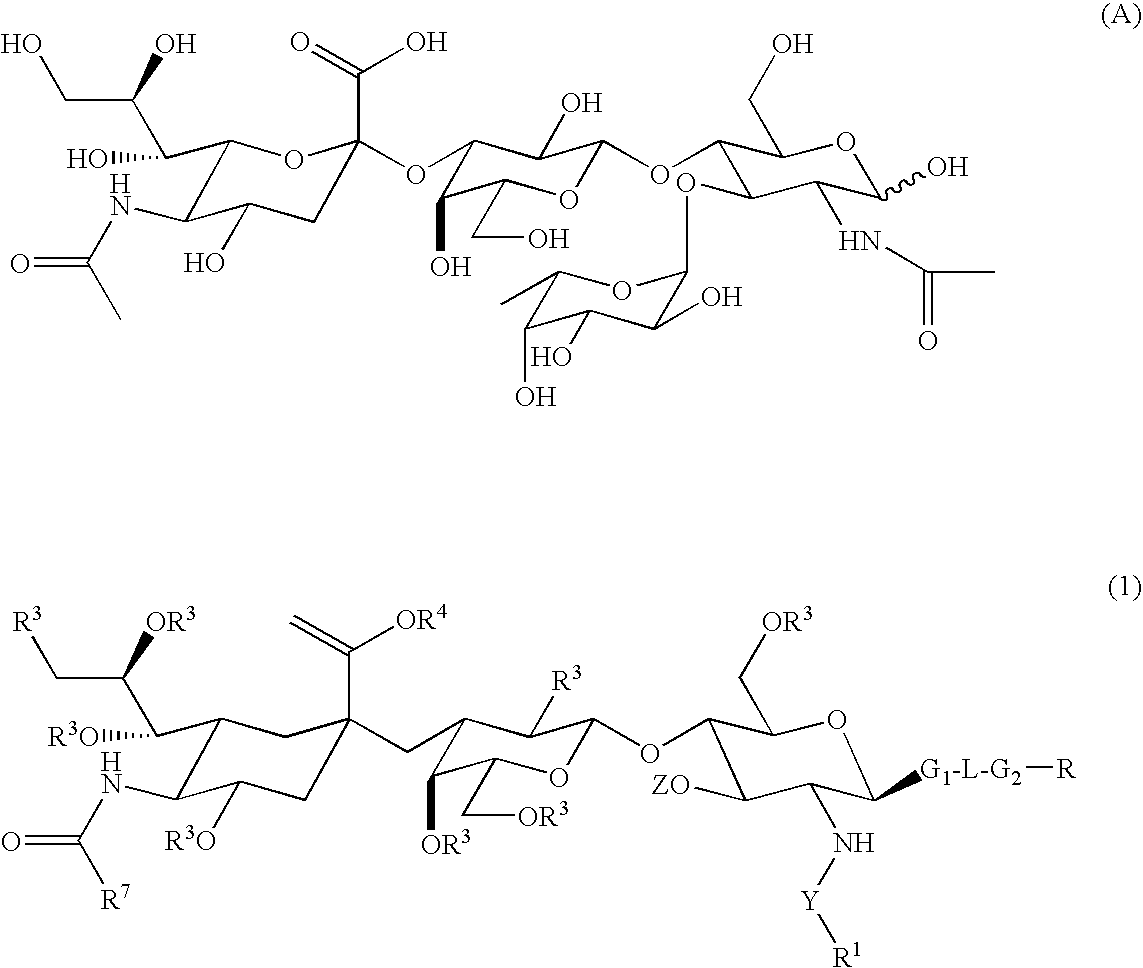
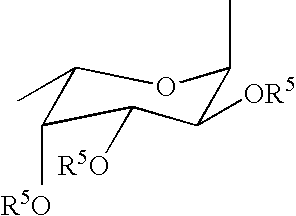
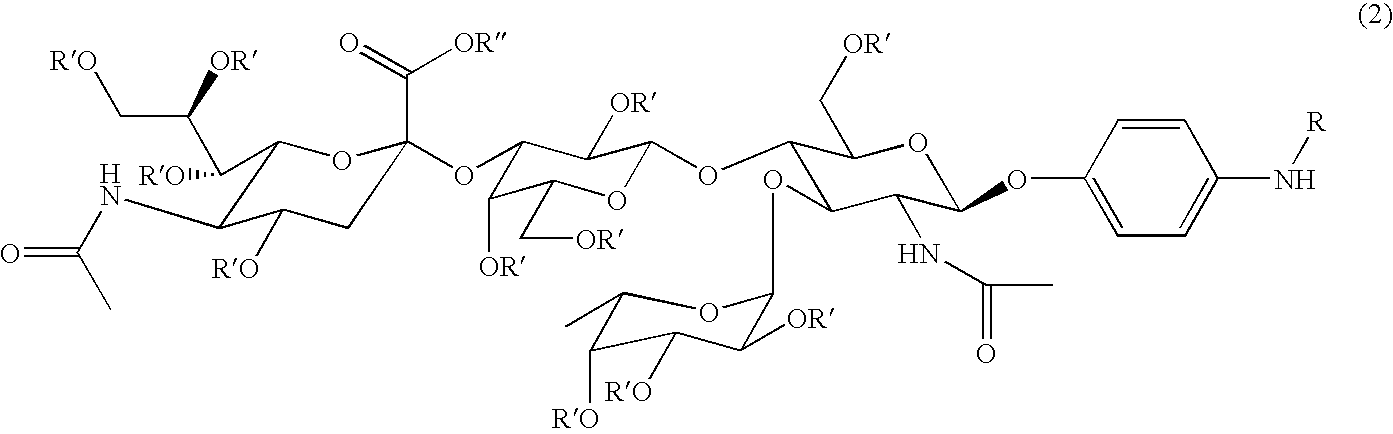
Linkable Lewis X Analogs
a sialyl lewis and analog technology, applied in the field of linkable sialyl lewis x, can solve the problems of unsuitable for many applications, including targeting, tissue damage instead of repair, etc., and achieve the effects of enhancing the ability of sialyl lewis to inhibit, increasing the usefulness of targeting applications, and increasing binding strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of p-Nitrophenyl 2-acetamido-4,6-benzylidine 2-deoxy-β-D-glucopyranoside (Compound 5)
[0109]The preparation of this compound was conducted as described in J. Roger, et al., Carbohydrate Res., 6:184-196 (1968), and is given below.
[0110]A mixture of p-nitrophenyl-2-acetamido-2-deoxy-β-D-glucopyranoside (compound 4) (10.0 g, 30 mmol), benzaldehyde dimethylacetal (10.0 g) and PTS.H2O (0.18 g) in DMF (50 mL) was stirred at 50° C. for about 3 h. TLC of the reaction mixture indicated complete conversion of the starting material to the benzylidine derivative. The mixture was cooled to RT and slowly added to a stirred ice-water (700 mL). The white solid formed was collected by filtration, washed with water and dried in vacuo to give p-nitrophenyl 2-acetamido-4,6-benzylidine 2-deoxy-β-D-glucopyranoside (compound 5) (12.2 g, yield 97%). TLC analysis (silica gel, 5% MeOH in CH2Cl2) showed a single spot (Rf 0.5, compared to 0.25 for the starting material) thus indicating that the produc...
example 2
Synthesis of 2,3,4-Tri-O-benzyl-1-O-p-nitrobenzoyl-α-L-fucopyranose (Compound 8)
[0111]The preparation of this compound was conducted as described in M. Dejter-Juszynski and H. M. Flowers, Carbohydrate Res., 18:219-216 (1971), and is given below.
[0112]To a cooled solution (5° C.) of 2,3,4-tri-O-benzyl-L-fucopyranose (compound 6) (6.0 g, 13.45 mmol) in dry CH2Cl2 (100 mL) was added pyridine (7.5 mL) followed by a slow addition of p-nitrobenzoyl chloride (compound 7) (3.15 g, 17 mmol) in portions over a 5 min period. After the addition, the mixture was stirred at 5° C. for 5-10 min then at RT for 18 h. The mixture was diluted with CH2Cl2 (100 mL) and the solution was washed successively with cold 1N HCl, water, cold aqueous NaHCO3, water, dried and concentrated to give a gummy product (9.0 g). TLC analysis (silica gel, hexane-EtOAc 3:1) indicated the presence of a major (Rf 0.6) and a minor (Rf 0.48) product, but no starting material. This could be due to a mixture of α and β isomers, ...
example 3
Synthesis of 2,3,4-tri-O-benzyl-α-L-fucopyranosyl bromide (Compound 9)
[0116]The preparation of this compound was conducted as described in M. Dejter-Juszynski and H. M. Flowers, Carbohydrate Res., 18:219-216 (1971), and is given below.
[0117]A saturated solution of HBr in CH2Cl2 (20.0 mL) was prepared by passing anhydrous HBr in CH2Cl2 at 0° C. for 30 min. This solution was added to a cold solution of the α-isomer of the 2,3,4-tri-O-benzylfucopyranose-p-nitrobenzoate (compound 8) (6.5 g, 11 mmol) in CH2Cl2 (150 mL) at 0-5° C., and the mixture was stirred at 5° C. for 30-40 min. The precipitated p-nitrobenzoic acid was filtered off, and the filtrate was successively washed with cold sat. NaHCO3 and water until neutral. The extract was dried (MgSO4) and the solvent removed at RT in vacuo to afford exclusively the α-fucopyranosyl bromide as a highly viscous liquid (5.3 g, yield 95%).
[0118]Similarly, treatment of the β-isomer of the fucopyranosyl-p-nitrobenzoate (compound 8) (583 mg, 1 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com