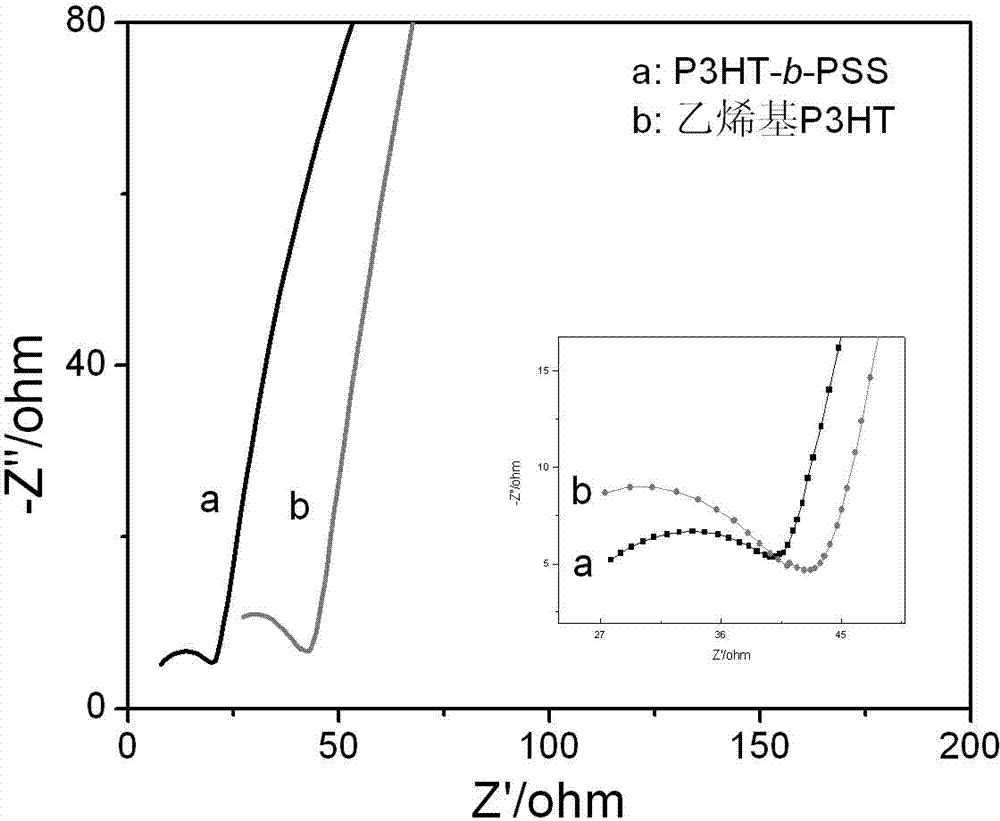
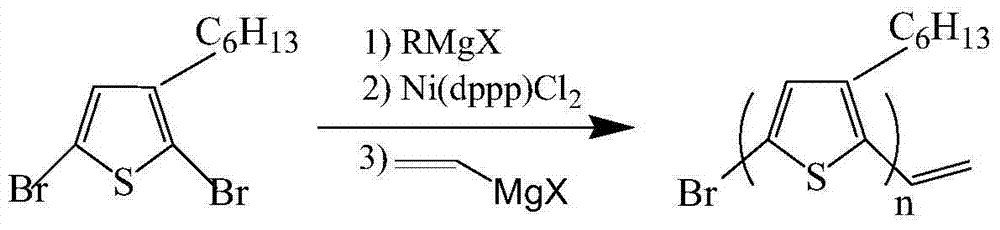Preparation method of self-doped thiophene polymer
A polymer and self-doping technology, which is applied in the field of preparation of self-doping thiophene block copolymers, can solve the problems of poor compatibility and dispersion, achieve good molding and processing performance, and be beneficial to material properties and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
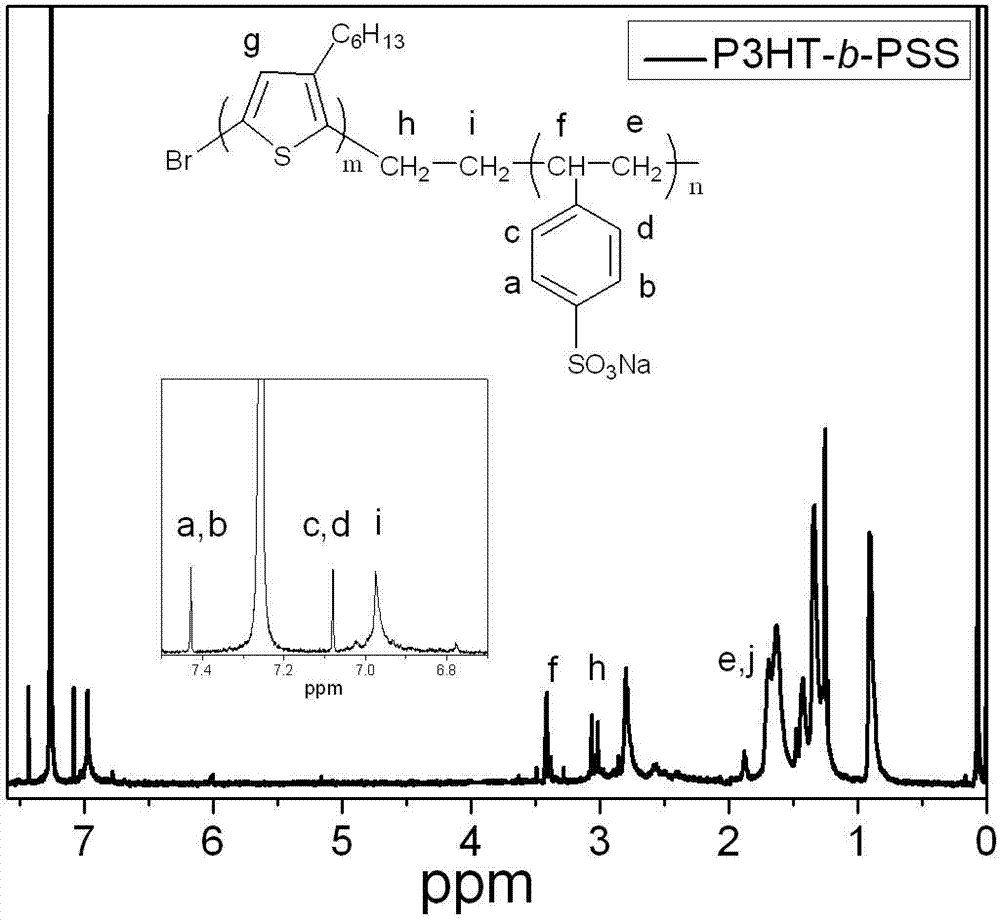
[0025]Embodiment 1: the synthesis of 3-hexylthiophene-b-sodium styrene sulfonate block copolymer
[0026] Step 1, preparation of ethylene-terminated poly-3-hexylthiophene:
[0027] With 100 mL of anhydrous tetrahydrofuran (relative density 0.8892) as the reaction solvent, add 3.26 g of 2,5-dibromo-3-hexylthiophene and 5 mL of tetrahydrofuran with butylmagnesium chloride at a concentration of 2 mol / L (M) (density 0.93 g / mL) Solution, the molar ratio of 2,5-dibromo-3-hexylthiophene and butylmagnesium chloride consumption is 1:1, the mass sum of 2,5-dibromo-3-hexylthiophene and butylmagnesium chloride and anhydrous tetrahydrofuran The mass ratio is 1:11; under the condition of nitrogen protection, the temperature is 0°C, and the reaction is stirred for 2 hours to obtain a reaction system; 0.065g of 1,3-bisdiphenylphosphinopropane nickel dichloride (Ni (dppp)Cl 2 ) catalyst for coupling reaction, Ni(dppp)Cl 2 The molar ratio of the dosage to the dosage of 2,5-dibromo-3-hexylthi...
Embodiment 2
[0035] Example 2: Synthesis of 3,4-ethylenedioxythiophene-b-sodium p-styrenesulfonate block copolymer
[0036] Step 1, the preparation of ethylene-terminated poly-3,4-ethylenedioxythiophene:
[0037] With 100 mL of anhydrous tetrahydrofuran (relative density 0.8892) as the reaction solvent, add 3 g of 2,5-dibromo-3,4-ethylenedioxythiophene and 5 mL of butane with a concentration of 2 mol / L (M) (density 0.93 g / mL) The tetrahydrofuran solution of butylmagnesium chloride, the molar ratio of the amount of 2,5-dibromo-3,4-ethylenedioxythiophene and the amount of butylmagnesium chloride is 1:1, 2,5-dibromo-3,4-vinyldioxythiophene The mass ratio of the sum of the mass of oxythiophene and butylmagnesium chloride to anhydrous tetrahydrofuran is 1:12; under the condition of nitrogen protection, the temperature is 0°C, and the reaction is stirred for 2 hours to obtain a reaction system; then add 0.108g of 1,3-bisdiphenylphosphinopropane nickel dichloride (Ni(dppp)Cl 2 ) catalyst for co...
Embodiment 3
[0044] Embodiment 3: Synthesis of 3-methylthiophene-b-ethylene sulfonate sodium block copolymer
[0045] Step 1, preparation of ethylene-terminated poly-3-methylthiophene
[0046] Prepared according to step 1 in Example 1, the dibromomonomer used is 2,5-dibromo-3-methylthiophene, and the alkylmagnesium halide Grignard reagent used has a concentration of 1mol / L (M) (density 0.941g / mL) tetrahydrofuran solution of isobutylmagnesium bromide;
[0047] Step 2, preparation of self-doping type 3-methylthiophene-b-sodium ethylene sulfonate block copolymer
[0048] It was prepared according to step 2 in Example 1, and the doping monomer used was sodium vinyl sulfonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com