Rasagiline orally disintegrating compositions
A technology of rasagiline mesylate and its composition, which is applied in the field of rasagiline orally disintegrating composition, which can solve the problems of high friability, increased cost, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
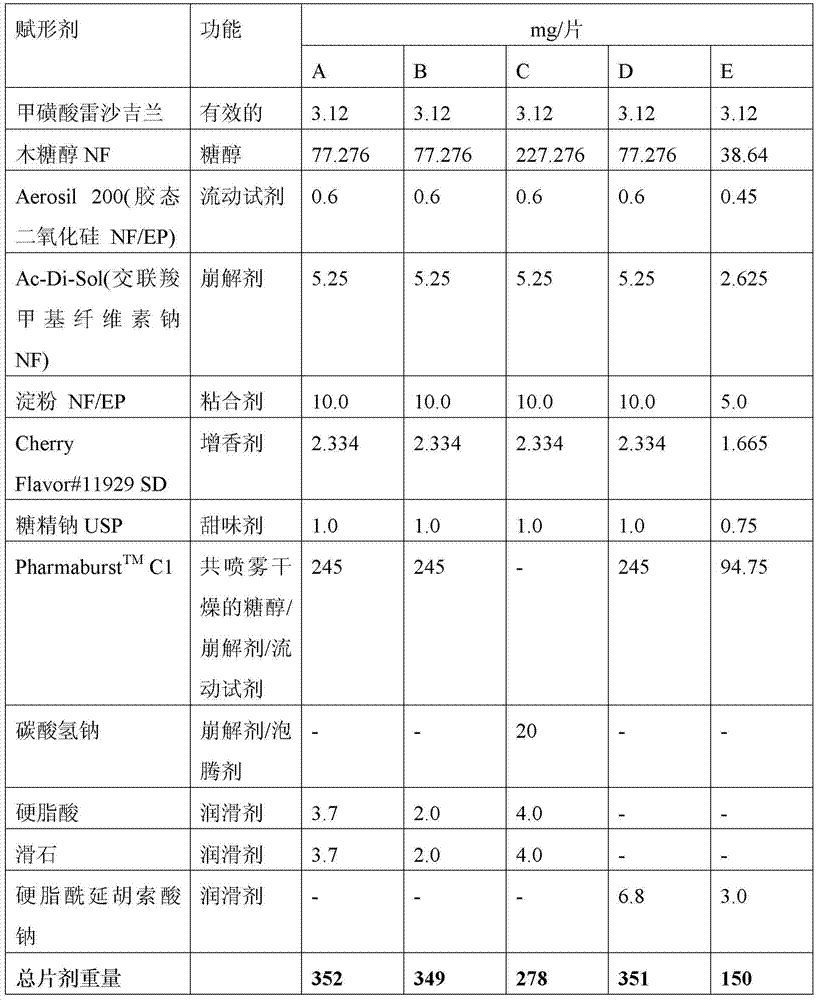
[0111] Formulation A was prepared using the following steps using the excipients in Table 1:
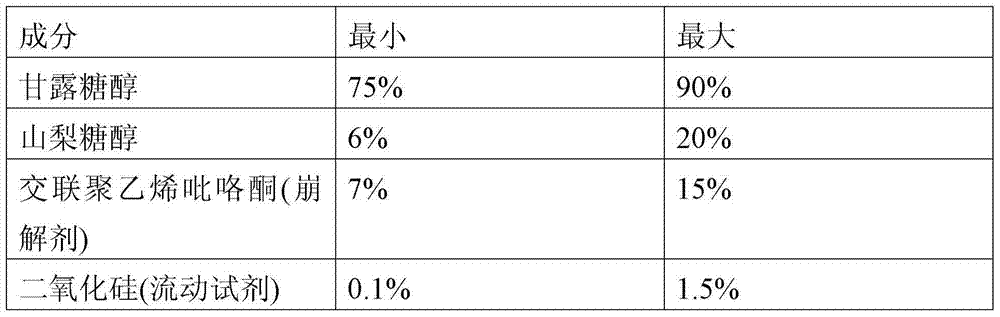
[0112] 1. Mix xylitol, aerosil 0.3mg / tablet, rasagiline mesylate, starch NF, Ac-Di-Sol, flavoring agent 1.34mg / tablet, and sodium saccharin 0.5mg / tablet for 5 minutes .
[0113] 2. Add Purified Water USP to the mixture from Step 1 and mix for 60 seconds.
[0114] 3. Dry the granules (outlet temperature: 44°C).
[0115] 4. Sieve the granules through a 0.6 mesh sieve.
[0116] 5. The granules are then mixed with 0.3 mg / tablet of aerosil, Pharmaburst TM , 0.5 mg / tablet of sodium saccharin, and 1 mg / tablet of cherry flavor were mixed for 15 minutes.
[0117] 6. The mixture from step 5 was then blended with stearic acid and talc for 5 minutes.
[0118] 7. Compress the tablet to a hardness of 5 kPa.
Embodiment 2
[0120] Formulation B was prepared using the following steps using the excipients in Table 1:
[0121] 1. Mix xylitol, aerosil 0.3mg / tablet, rasagiline mesylate, starch NF, Ac-Di-Sol, flavoring agent 1.34mg / tablet, and sodium saccharin 0.5mg / tablet for 5 minutes .
[0122] 2. Add Purified Water USP to the mixture from Step 1 and mix for 60 seconds.
[0123] 3. Dry the granules (outlet temperature: 44°C).
[0124] 4. Sieve the granules through a 0.6 mesh sieve.
[0125] 5. The granules are then mixed with 0.3 mg / tablet of aerosil, Pharmaburst TM , 0.5 mg / tablet of sodium saccharin, and 1 mg / tablet of cherry flavor were mixed for 15 minutes.
[0126] 6. The mixture from step 5 was then blended with stearic acid and talc for 5 minutes.
[0127] 7. Compress the tablet to a hardness of 6 kPa.
Embodiment 3
[0129] Formulation C was prepared using the following steps using the excipients in Table 1:
[0130] 1. Mix 77.276mg / tablet of xylitol, 0.3mg / tablet of aerosil, rasagiline mesylate, starch NF, Ac-Di-Sol, 1.34mg / tablet of flavoring agent, and 0.5mg / tablet of The sodium saccharin was mixed for 5 minutes.
[0131] 2. Add Purified Water USP to the mixture from Step 1 and mix for 60 seconds.
[0132] 3. Dry the granules (outlet temperature: 44°C).
[0133] 4. Sieve the granules through a 0.6 mesh sieve.
[0134] 5. The granules were then mixed with 0.3 mg / tablet of aerosil, sodium bicarbonate, 150 mg / tablet of xylitol, 0.5 mg / tablet of sodium saccharin, and 1 mg / tablet of cherry flavor for 15 minutes.
[0135] 6. The mixture from step 5 was then blended with stearic acid and talc for 5 minutes.
[0136] 7. Compress the tablet to a hardness of 4 kPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com