Glycosylated maltose binding protein, and preparation method and application thereof
A technology for binding proteins and maltose, applied in biochemical equipment and methods, chemical instruments and methods, and botanical equipment and methods, etc., can solve problems such as difficulty in operation, heterogeneity of glycoproteins, complicated steps, etc., and achieve simplified steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of maltose-binding protein containing pentapeptide glycosylation sequence (DQNAT)
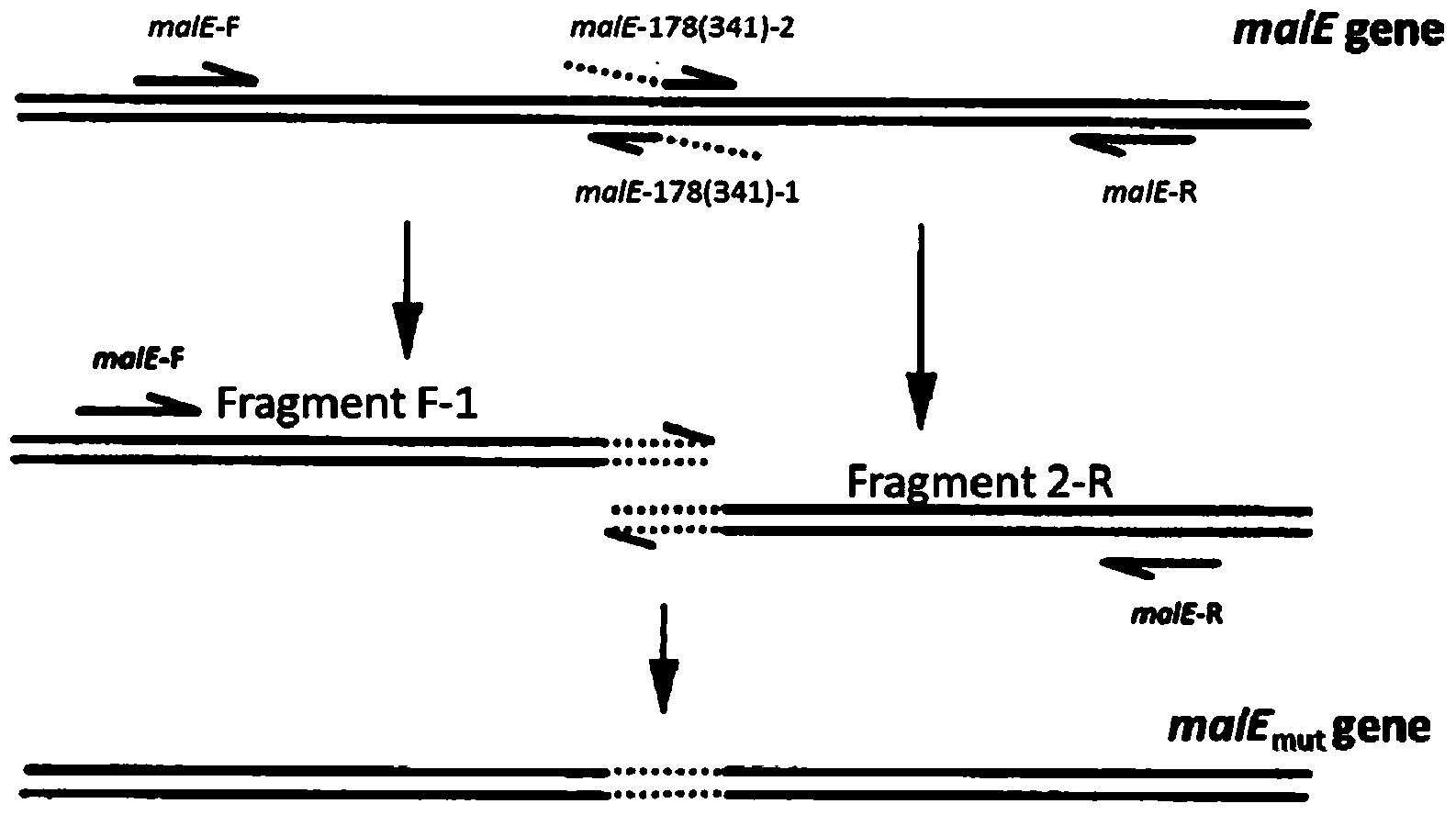
[0048]According to the central dogma, DNA is transcribed into RNA and RNA is translated into protein. Therefore, the malE gene is modified at the DNA level, the nucleotide sequence encoding DQNAT is inserted, and then the modified malE gene is expressed to obtain the MBP containing the pentapeptide glycosylation site. Take the malE gene in the plasmid pMAL-p5X as a template, and use the malE-178-1, malE-178-2, malE-341-1, malE-341-2, malE-F, and malE-R listed in Table 1 as The primers were inserted into the pentapeptide glycosylation sequence (DQNAT) at the N178 and N341 positions of MBP respectively twice.
[0049] The steps are as shown in the figure, taking inserting DQNAT at bit N178 of the MBP as an example, the specific instructions are as follows:
[0050] 1) The 5' end of the primer malE-178-1 is complementary to the 5' end of malE-178-2, which is responsible for the ...
Embodiment 2
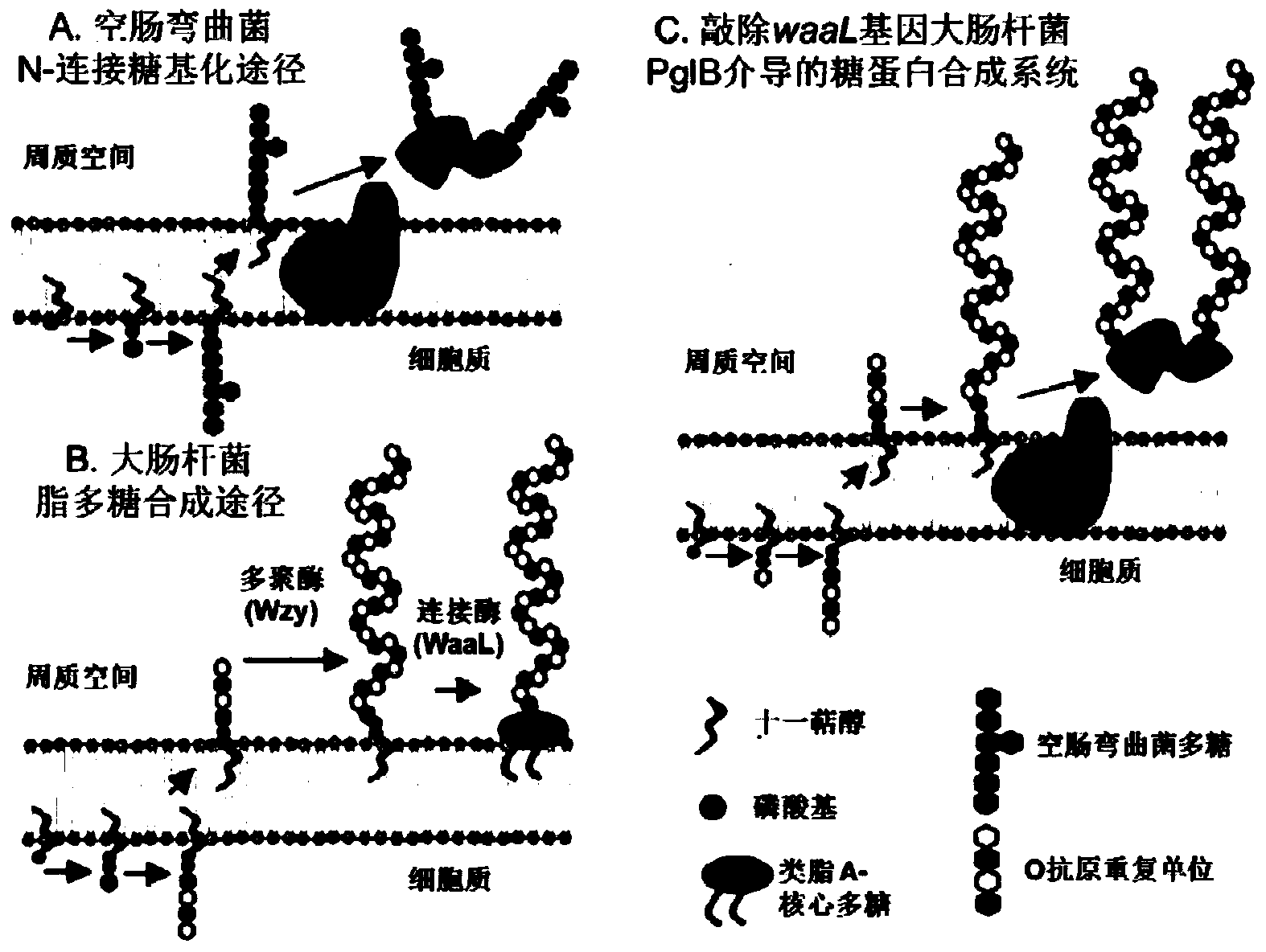
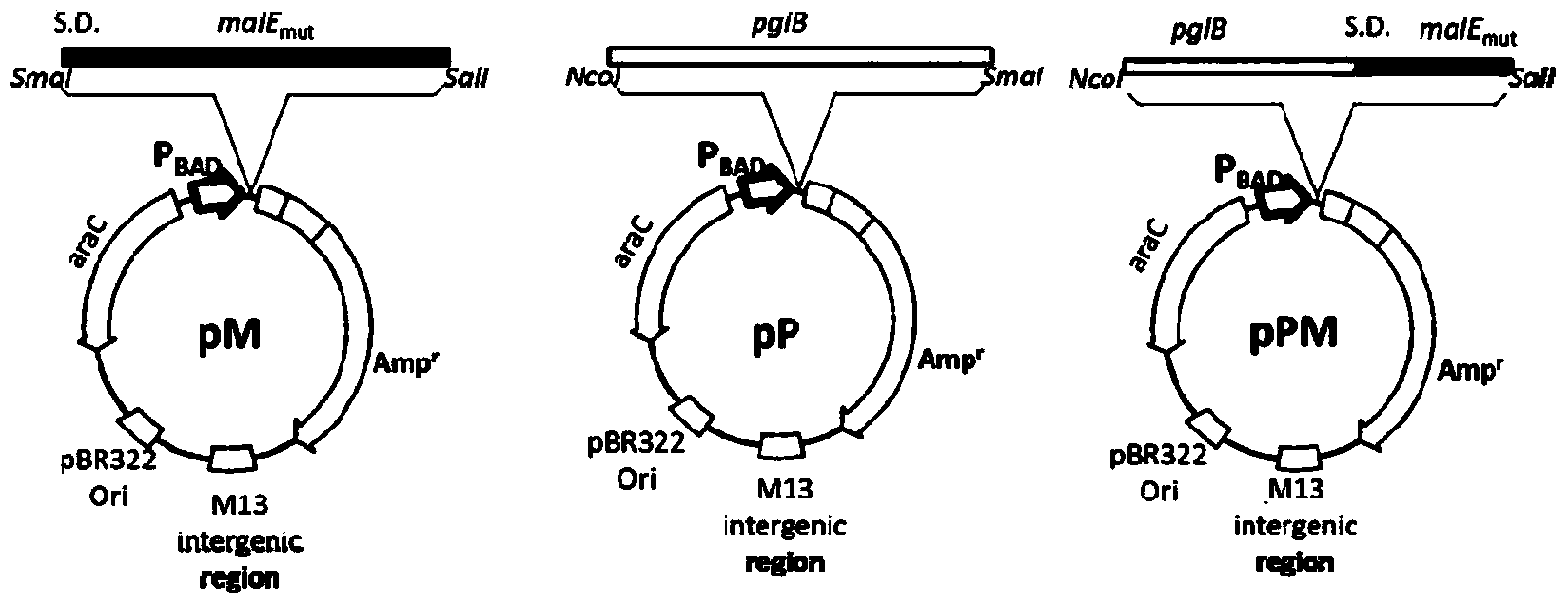
[0057] Construction of Escherichia coli Glycoprotein Synthesis System Mediated by PglB
[0058] Take the model bacteria Escherichia coli O86:K61:B7 as an example to describe the specific steps in detail, as follows:
[0059] Knockout of the waaL gene in E. coli O86:K61:B7. For the knockout method, refer to the λ-Red recombination system. Knockout primers k-waaL-F, k-waaL-R and detection primers t-waaL-F, t-waaL-R were used; the plasmids used were pKD46, pKD4 and pCP20. Knockout validation results such as Figure 4 shown. Some conditions are briefly described as follows:
[0060] (1) Transformation of pKD46 and induced expression of the Red recombination system: transform the plasmid pKD46 encoding the Red recombination gene into E.coli O86:K61:B7 by chemical method, and inoculate it in LB liquid medium containing 100 μg / mL Amp Incubate overnight at 30°C and 250 rpm. Then transfer 1% inoculum to 50mL fresh LB liquid medium containing 1‰Amp, culture for one hour OD600=0.2~...
Embodiment 3
[0069] The preparation method of maltose binding protein expression gene malE-Mut comprises the following steps:
[0070] (1) Design a pair of chimeric primers whose sequences are:
[0071] malE-178-1: 5'TGTTGCGTTCTGGTC,
[0072] malE-178-2: 5'GACCAGAACGCAACA;
[0073] Design the most distant upstream and downstream primers for a pair of template genes, respectively:
[0074] k-waaL-F: CTCGAGAAAAAAAACTGGATAGCGTACTGGAACAGAGCTGTGTAGGCTGGAGCTGCTTC and
[0075] k-waaL-R:TTACTTGTTTTTCATCGCTAATAATAAGCCGGCGTAAACATGGGAATTAGCCATGGTCC;
[0076] Then use malE-178-1 and k-waaL-F as primers and malE gene as template to synthesize PCR fragment k-waaL-F-malE-178-1; then use malE-178-2 and k-waaL-R As a template, synthesize the fragment malE-178-2-k-waaL-R;
[0077] Use the mixture of PCR fragments k-waaL-F-malE-178-1 and malE-178-2-k-waaL-R as a common template, and use the malE gene as a template to synthesize a PCR product and insert pentapeptide sugar at position N178 The malE mutan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com