Method for establishing heart disease drug screening model
A heart disease and drug technology, applied in the field of biomedicine, can solve the problems of different effects and side effects, and achieve the effect of wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, the preparation of IPS cell
[0030] 1.1. Four transcription factors, Oct3 / 4, Sox2, c-Myc and klf4, are coated with lentivirus
[0031] Four transcription factors, Oct3 / 4, Sox2, c-Myc and klf4, were amplified from total mouse cDNA by PCR. The primer sequences used in PCR amplification are as follows:
[0032] Oct3 / 4: 5' Primer: 5'-ATGGCTGGACACCTGGCTTCAGAC-3'
[0033] 3' Primer: 5'-GTTTGAATGCATGGGAGAGCCCAG-3'
[0034] Sox2: 5' Primer: 5'-ATGTATAACATGATGGAGACGGAG-3'
[0035] 3' Primer: 5'-CATGTGCGACAGGGGCAGTG-3'
[0036] c-Myc: 5' Primer: 5'-ATGCCCCTCAACGTGAACTTCAC-3'
[0037] 3' Primer: 5'-TGCACCAGAGTTTCGAAGCTGTTC-3'
[0038] klf4: 5' Primer: 5'-ATGAGGCAGCCACCTGGCG-3'
[0039] 3' Primer: 5'-AAAGTGCCTCTTCATGTGTAAGG-3'.
[0040] The PCR amplification products of the four transcription factors were respectively inserted into the lentiviral vector lenti-ef1a-EGFP (purchased from Invitrogen), and co-transfected into virus packaging cells, namely 293T cel...
Embodiment 2
[0050] Example 2, Identification of IPS cell totipotency
[0051] In order to verify the pluripotency of the IPS cells obtained in Example 1 and make them naturally differentiate to form embryoid bodies (EBs), the embryoid bodies were sliced and stained with three germ layer-specific Marker antibodies to observe the embryoid bodies (EBs) of each germ layer. Express the situation. Among them, endoderm-specific markers are AFP and Sox17; mesoderm-specific markers are SMA and a-actin; ectoderm-specific markers are Nestin and Tuj1. The result is as figure 1 As shown, colony formation can be observed.
Embodiment 3
[0052] Example 3, IPS cells are directed to differentiate into cardiomyocytes
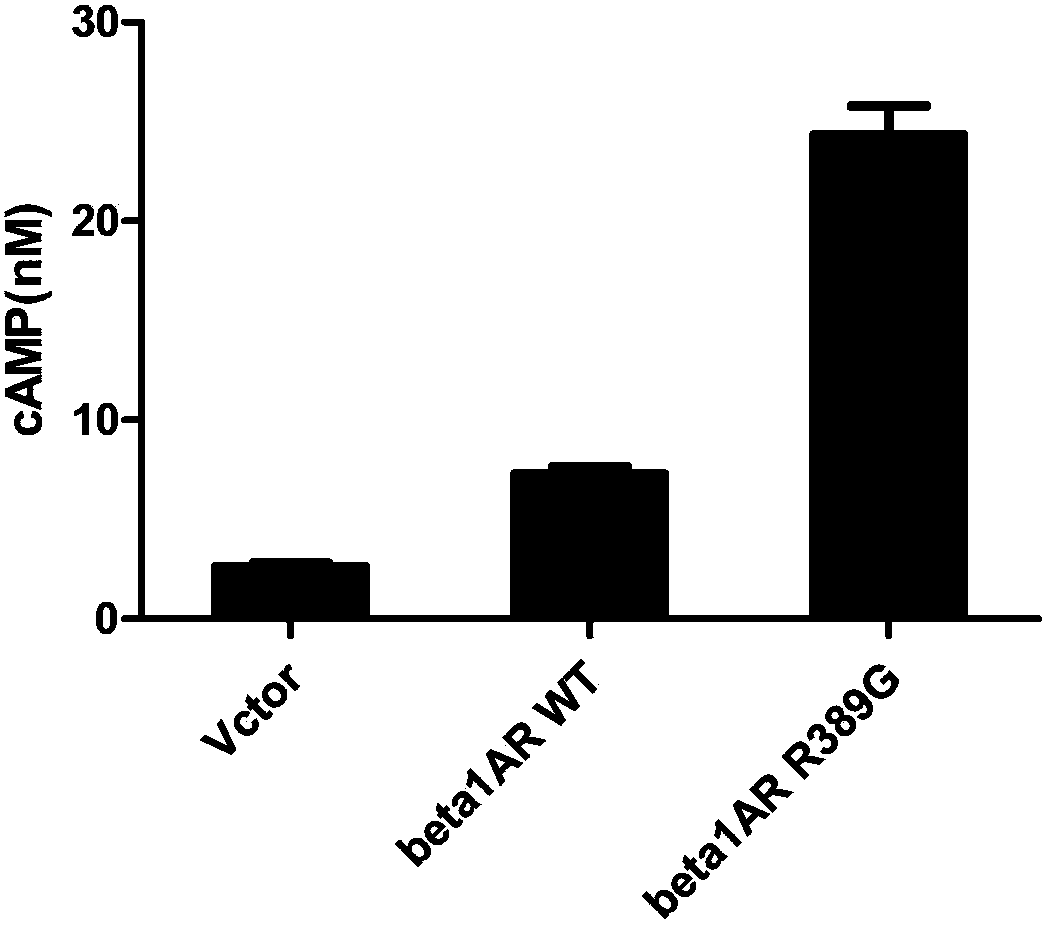
[0053] The wild-type beta1AR and beta2AR genes were mutated into beta1ARR389G and beta2AR G16R genes by site-directed mutagenesis, and then the wild-type beta1AR and beta2AR genes and mutant beta1AR R389G and beta2AR G16R genes were inserted into the lentiviral vector lenti-ef1a-EGFP ( purchased from Invitrogen), and co-transfected virus packaging cells, namely 293T cells (from Duke University, USA). Cultivate for 48-72hrs, and collect the supernatant culture solution containing the virus. Pass the supernatant through a 45 μm filter, then transfer to a sterile storage tube and save for later use.
[0054] The IPS cells obtained in Example 1 were infected with the above-mentioned lentivirus, and the experiment was divided into 3 groups:
[0055] a. Blank control group: lentivirus carrying green fluorescent protein;
[0056] b. Transfection of wild-type beta1AR and mutant beta1AR R389G into beta1A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com