Humanized M-CSF mice
A humanized and mouse technology, applied in the field of humanized M-CSF mice, can solve problems such as poor myeloid differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1: Cell Preparations, Analytical Methods, and Assays
[0144] CD34 +Cell isolation and transplantation. Human fetal liver samples were obtained from the Human Fetal Liver Tissue Collection at Albert Einstein College of Medicine, Bronx, NY and from Advance Biosciences Resources, Inc., Alameda, CA. All experiments involving human tissue were performed under the approval of the Yale Human Investigations Committee.
[0145] To isolate human CD34 + For cells, the fetal liver tissue was rinsed once with PBS, cut into small pieces, and treated with collagenase D (100 ng / mL) at 37° C. for 45 minutes. Single cell suspensions were prepared and mononuclear cells were isolated using density gradient centrifugation (lymphocyte separation medium, MP biomedicals). After treatment of cells with anti-human CD34 beads, followed by MACS TM technology (Miltenyi Biotech) to isolate CD34 + cell.
[0146] For transplantation, newborn puppies (day 1 of birth) were sublethally ir...
Embodiment 2
[0160] Example 2: Genetically Modified Mice for Grafting
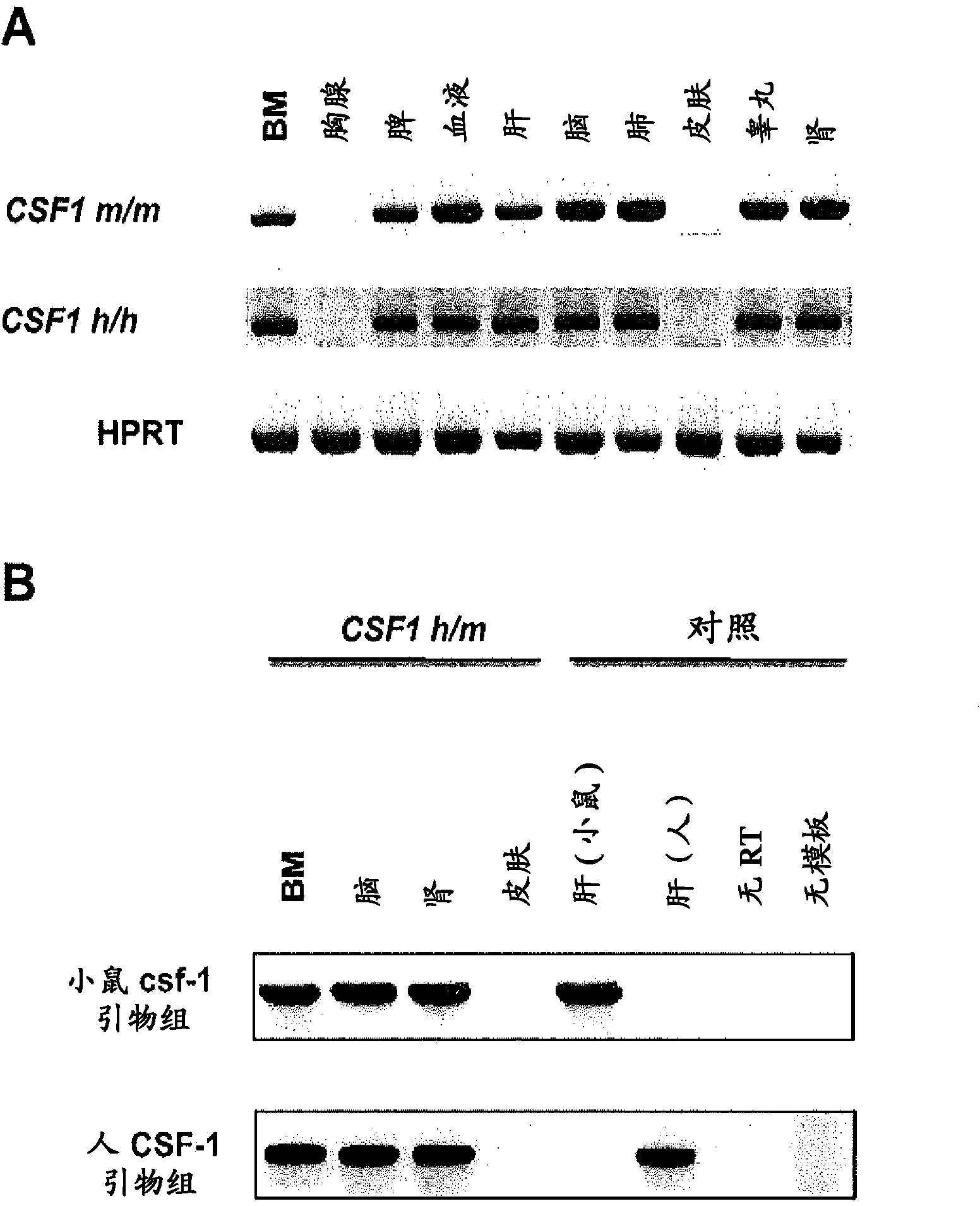
[0161] Human M-CSF knock-in strategy. use The technical construction is used to use human M-CSF nucleic acid sequence in a single targeting step ( Allele identification number 5093) to replace the targeting construct of the mouse M-CSF nucleic acid sequence, as previously described (Valenzuela et al. (2003) High-throughput engineering of the mouse genome coupled with high-resolution expression analysis, Nat.Biotechnol .21:652-659). Mouse and human M-CSF DNA were obtained from bacterial artificial chromosome (BAC) RPCI-23, clone 373B18 and from BAC RPCI-11, clone 101M23, respectively. Briefly, linearized targeting constructs generated by gap repair cloning were electroporated into RAG2 + / - gamma c - / -In mouse embryonic stem (ES) cells generated from a commercial V17ES cell line (BALB / c x129F1), the linearized targeting construct contained 633nt downstream of non-coding exon 9 extending from exon 2 The mouse M-CS...
Embodiment 3
[0168] Example 3: Differentiation of Human Monocytes / Macrophages in Humanized M-CSF Mice
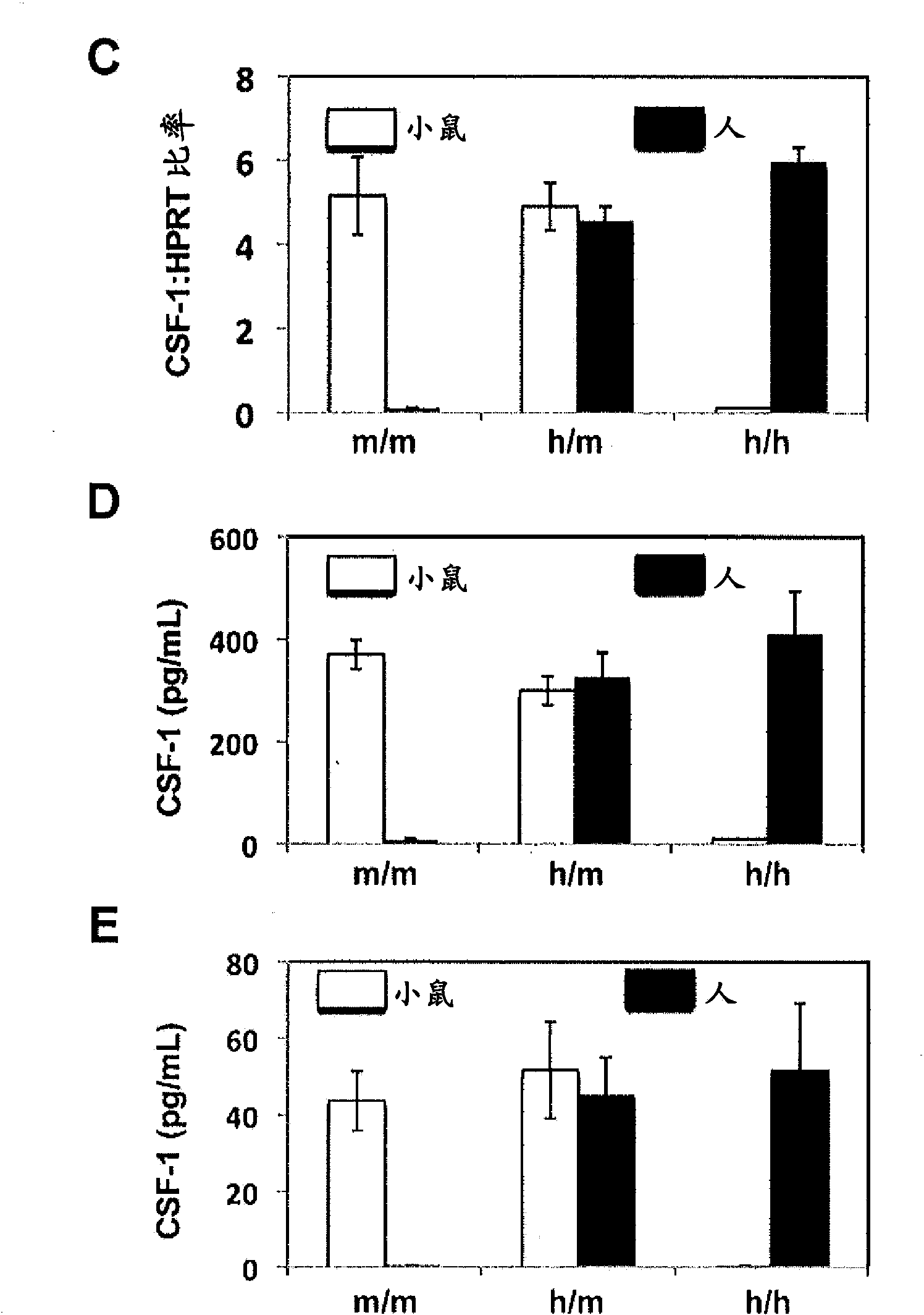
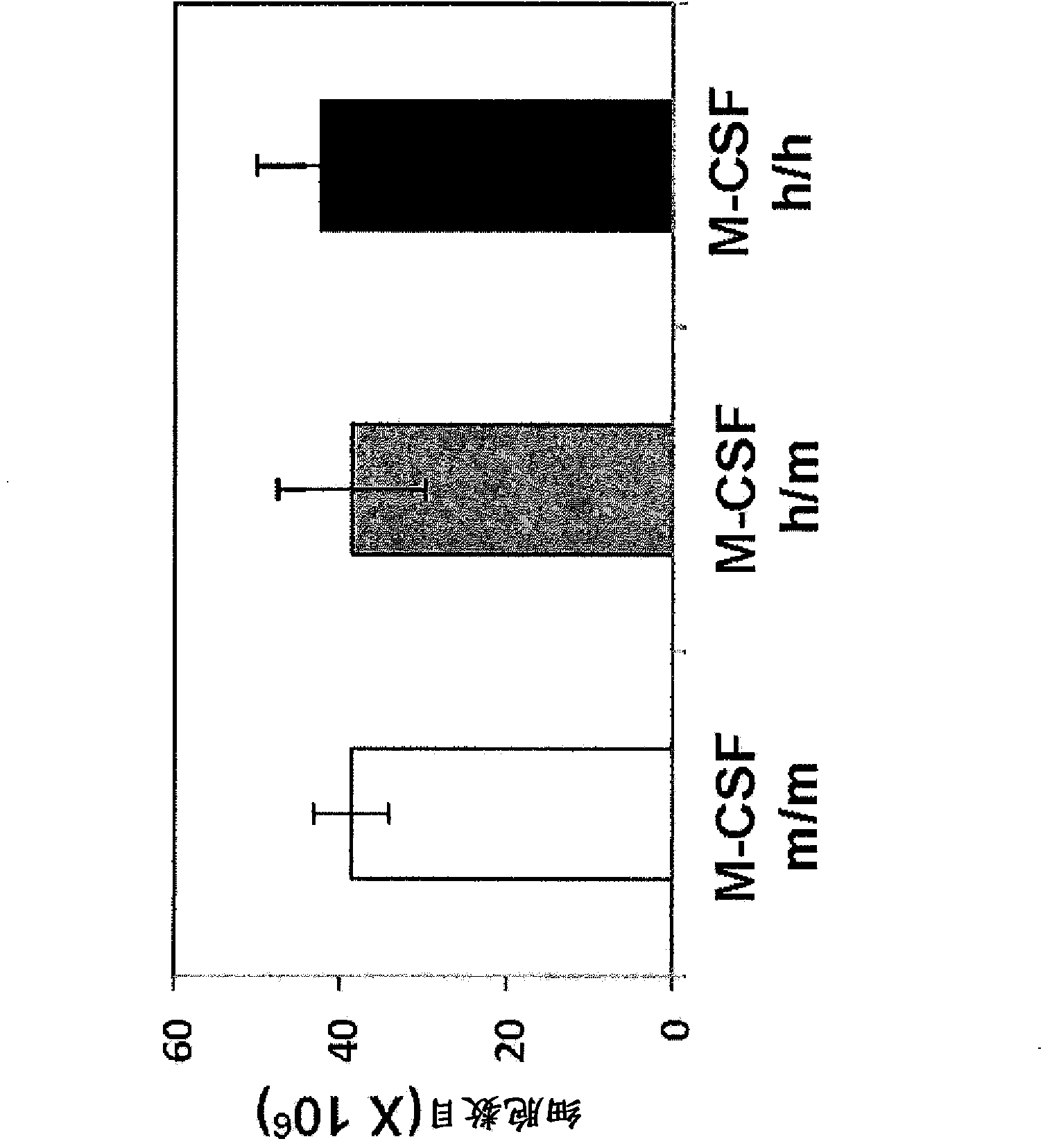
[0169] To assess the impact of M-CSF humanization, sublethally irradiated neonatal Rag2 - / - gamma c - / - M-CSF m / m ,Rag2 - / - gamma c - / - M-CSF h / m and Rag2 - / - gamma c - / - M-CSF h / h Intrahepatic (i.h) transplantation of pups approximately 2x10 5 purified human fetal liver CD34 + cell. Recipients were then bled at 8 weeks post-transplantation to confirm donor-derived cells (based on human CD45 expression). Twelve weeks after transplantation, recipients were sacrificed and their BM, SP and PB were harvested. Analysis revealed with M-CSF m / m Mice compared to M-CSF h / m and M-CSF h / h CD14 in BM, SP and PB of both mice + CD33 + Increased relative and absolute frequencies of monocyte / macrophage lineage cells ( Figure 3A -C). Although M-CSF h / m Mice exhibit elevated CD14 + CD33 + cell frequency, but CD14 + CD33 + The greatest frequency of cells exists in M-CSF h / h in mice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com