Free alkali tolterodine filming hydrogel preparation and preparation method thereof through later drug loading
A gel preparation and gel technology are applied in the improvement of tolterodine pharmaceutical dosage forms, the preparation of tolterodine film-forming hydrogels, and the field of free-base tolterodine film-forming hydrogel preparations. Improve physical properties and fine texture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare the gel (according to 10g) according to the following prescription:
[0034] Tolterodine tartrate 0.584g, Carbomer 980 0.1g, hydroxypropylcellulose (HPC) 0.05g, ethyl acetate 200μl, hydroxypropylmethylcellulose (HPMC) 0.2g, ethanol 4ml, Tween 80 0.1 g, the balance is distilled water.
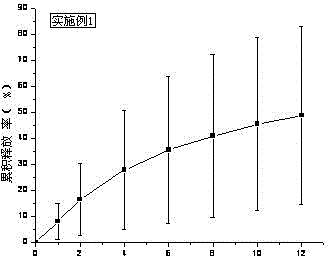
[0035] Treat tolterodine tartrate lye as KOH. Carbomer 980, HPC, and HPMC were weighed and added to a beaker, swelled with 6ml of water for 12 hours, added Tween 80, ethyl acetate and the rest of ethanol, stirred, added water to 10g, stirred until the gel was uniform and transparent, and put into a vial Store sealed at room temperature. In vitro transdermal cumulative release rate-time curve see figure 1 .
Embodiment 2
[0037] Prepare the gel (according to 10g) according to the following prescription:
[0038] Tolterodine tartrate 0.584g, Carbomer 980 0.1g, hydroxypropylcellulose (HPC) 0.05g, ethyl acetate 500μl, hydroxypropylmethylcellulose (HPMC) 0.15g, ethanol 4ml, Tween 80 0.1 g, the balance is distilled water.
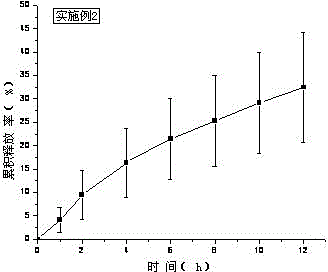
[0039] Treat tolterodine tartrate lye as KOH. Carbomer 980, HPC, and HPMC were weighed and added to a beaker, swelled with 6ml of water for 12 hours, added Tween 80, ethyl acetate and the rest of ethanol, stirred, added water to 10g, stirred until the gel was uniform and transparent, and put into a vial Store sealed at room temperature. In vitro transdermal cumulative release rate-time curve see figure 2 .
Embodiment 3
[0040] Example 3 Tolterodine tartrate gel preparation
[0041] Prepare the gel (according to 10g) according to the following prescription:
[0042] Tolterodine tartrate 0.584g, Carbomer 980 0.1g, hydroxypropylcellulose (HPC) 0.05g, ethyl acetate 100μl, hydroxypropylmethylcellulose (HPMC) 0.25g, ethanol 4ml, Tween 80 0.15 g, the balance is distilled water.
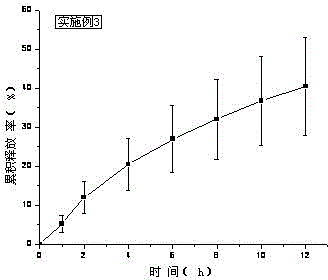
[0043] Treat tolterodine tartrate lye as KOH. Carbomer 980, HPC, and HPMC were weighed and added to a beaker, swelled with 6ml of water for 12 hours, added Tween 80, ethyl acetate and the rest of ethanol, stirred, added water to 10g, stirred until the gel was uniform and transparent, and put into a vial Store sealed at room temperature. In vitro transdermal cumulative release rate-time curve see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com