Tolterodine film-forming hydrogel preparation and preparation method thereof
A kind of technology of gel preparation, regulator, be applied in [0010] The present invention also provides tolterodine into, tolterodine film-forming hydrogel preparation, the field of improvement of tolterodine pharmaceutical dosage form, achieves good compatibility, The effect of smooth drug release and ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Prepare the gel (according to 10g) according to the following prescription:
[0046] Tolterodine Tartrate 0.5844g, Carbomer 980 0.1g, Hydroxypropylcellulose (HPC) 0.05g, Triethanolamine 0.5997g, Hypromellose (HPMC) 0.1g, Ethanol 4ml, Tween 0.1g , and the balance is distilled water.
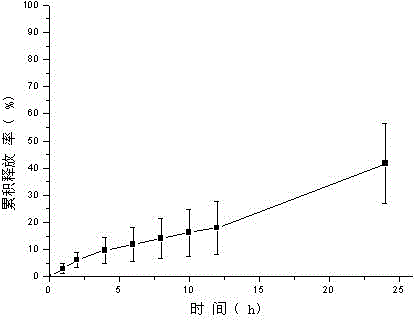
[0047] Weigh tolterodine tartrate, carbomer 980, HPC, and HPMC and add them into a beaker, add ethanol, triethanolamine, and Tween 80, stir, add water to 10g, stir until the gel is uniform and transparent, and put it into a vial Store sealed at room temperature. In vitro transdermal cumulative release rate-time curve see figure 1 .
Embodiment 2
[0049] Prepare the gel (according to 10g) according to the following prescription:
[0050] Tolterodine Tartrate 0.5844g, Carbomer 980 0.1g, Hydroxypropylcellulose (HPC) 0.05g, Triethanolamine 0.5997g, Hypromellose (HPMC) 0.15g, Ethanol 3.2g, Tween 0.1 g, the balance is distilled water.
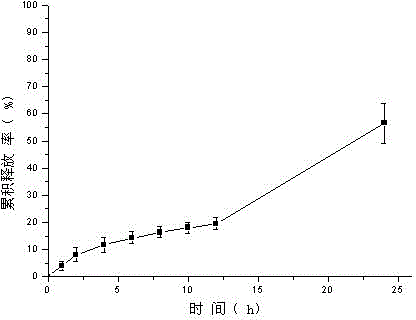
[0051] Weigh tolterodine tartrate, carbomer 980, HPC, and HPMC and add them to a beaker, add ethanol, triethanolamine, and Tween 80, stir, add water to 10g, stir until the gel is uniform and transparent, and put it into a vial Store sealed at room temperature. In vitro transdermal cumulative release rate-time curve see figure 2 .
Embodiment 3
[0052] Example 3 Tolterodine tartrate gel preparation
[0053] Prepare the gel (according to 10g) according to the following prescription:
[0054] Tolterodine Tartrate 0.8767g, Carbomer 980 0.1g, Hydroxypropylcellulose (HPC) 0.05g, Triethanolamine 0.783g, Hypromellose (HPMC) 0.1g, Ethanol 3.2g, Tween 0.1 g, the balance is distilled water.
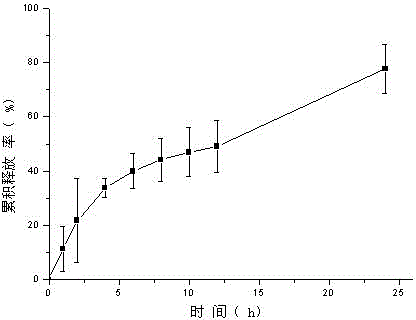
[0055] Weigh tolterodine tartrate, carbomer 980, HPC, and HPMC and add them into a beaker, add ethanol, triethanolamine, and Tween 80, stir, add water to 10g, stir until the gel is uniform and transparent, and put it into a vial Store sealed at room temperature. In vitro transdermal cumulative release rate-time curve see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com