Biomarkers for lung cancer
A biomarker, non-small cell lung cancer technology, applied in the direction of biological testing, biochemical equipment and methods, microbial determination/testing, etc., can solve the problems of not recommending patients, supplementing chemotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
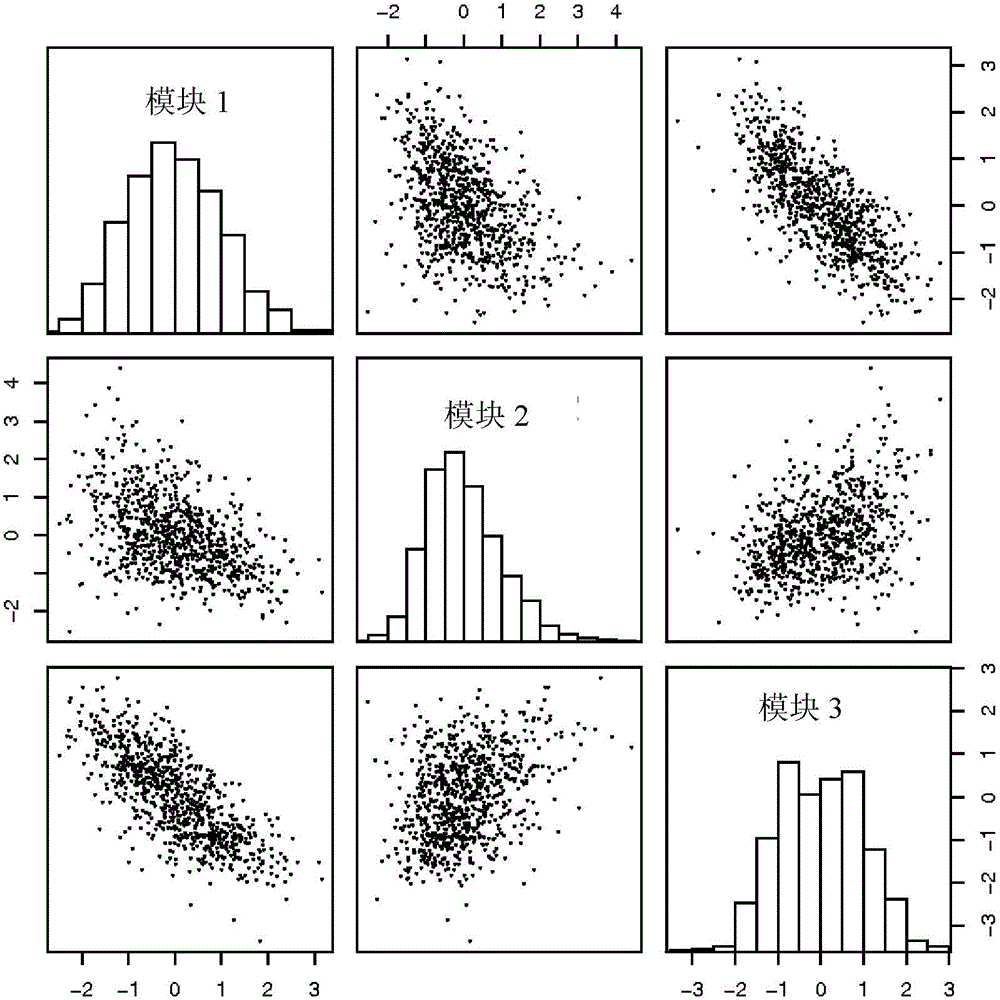
[0099] Combinations of the Novartis dataset and various publicly available datasets from the lung cancer patient cohort were studied. Descriptions of patient selection criteria and clinical characteristics can be found in the respective original articles of publicly available datasets (see below). For the Novartis dataset, 412 patient samples were collected from NSCLC patients who had undergone surgical resection. A standard staging procedure including CT scan, FDG-PET and MRI of suspicious lymph nodes (>1 cm in CT) was completed. NSCLC histological analysis was performed to determine whether NSCLC was squamous cell carcinoma, adenocarcinoma, or other carcinomas such as BAC. TNM-based staging is also performed to determine whether NSCLC is stage I or II. Banks of fresh-frozen tissues were established for genomic analysis. The primary endpoint was overall survival. Overall survival refers to the time (in years) from the first surgery and can be defined by a time period free...
Embodiment 2
[0130] Typical applications of the biomarkers disclosed in Tables 1, 2 and / or 3 with respect to lung cancer patients.
[0131] 1. Surgery to remove the lesion in a patient diagnosed with lung cancer with a small, operable primary tumor. Part of the tumor tissue is examined by standard pathology procedures, such as determination of tumor size, tumor histological type (eg, adenocarcinoma, squamous cell carcinoma, or other types). Tumor staging was determined using standard guidelines based on tumor size, presence of lymph node metastases, or metastases to other distant sites. Information obtained from standard clinicopathological measurements may improve and enhance the prognostic and predictive applications of the present invention, but is not required and is not a component.
[0132] 2. Part of the tumor tissue is used as the source material of the present invention in the form of frozen, paraffin-embedded or fresh tissue. Whole-transcriptome RNA extraction was performed on ...
Embodiment 3
[0149] Application of the label in formalin-fixed paraffin-embedded (FFPE) tumor material.
[0150] After tissue preparation and raw data preprocessing process suitable for each platform, the signatures (gene sets and formulas used to generate risk scores) protected by the present invention can be directly applied to data from technical platforms such as Affymetrix, qNPA or nanoString. express data.
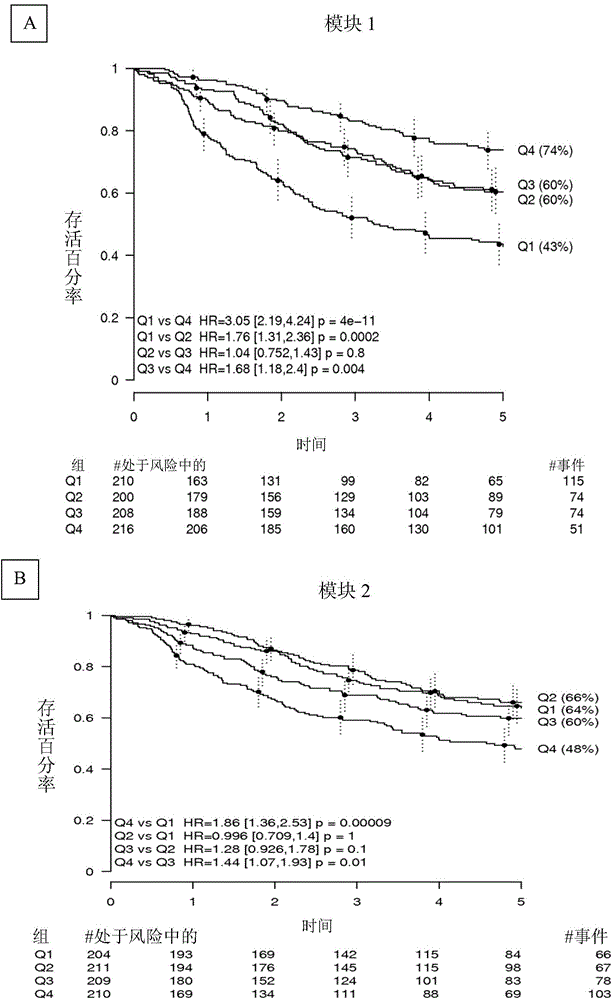
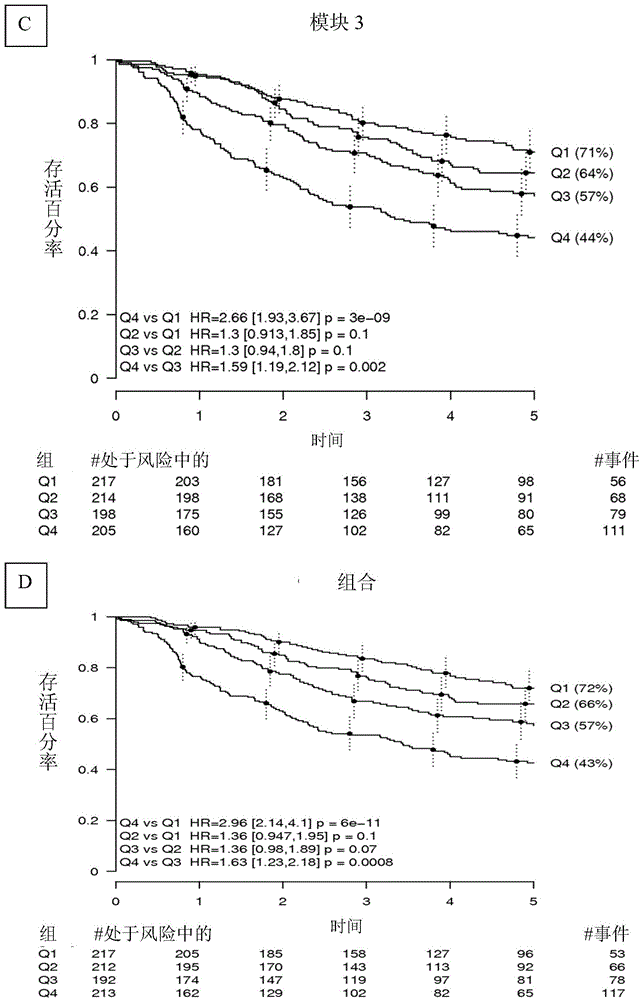
[0151] attached Figure 9 Survival efficacy is shown in a publicly available dataset from Anderson Cancer Center (Xie et al., 2011 Clin Cancer Res 17:5705-14; Gene Expression Omnibus accession GSE29013), obtained from FFPE material. Here, significant prognostic power was still observed, although Affymetrix data from FFPE were generally of lower quality (higher noise level, larger missing calls for expression values).
[0152] To evaluate whether the tagged risk score could provide the same prognostic value in qNPA and nanoString data from FFPE as in Affymetrix from fresh-frozen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com