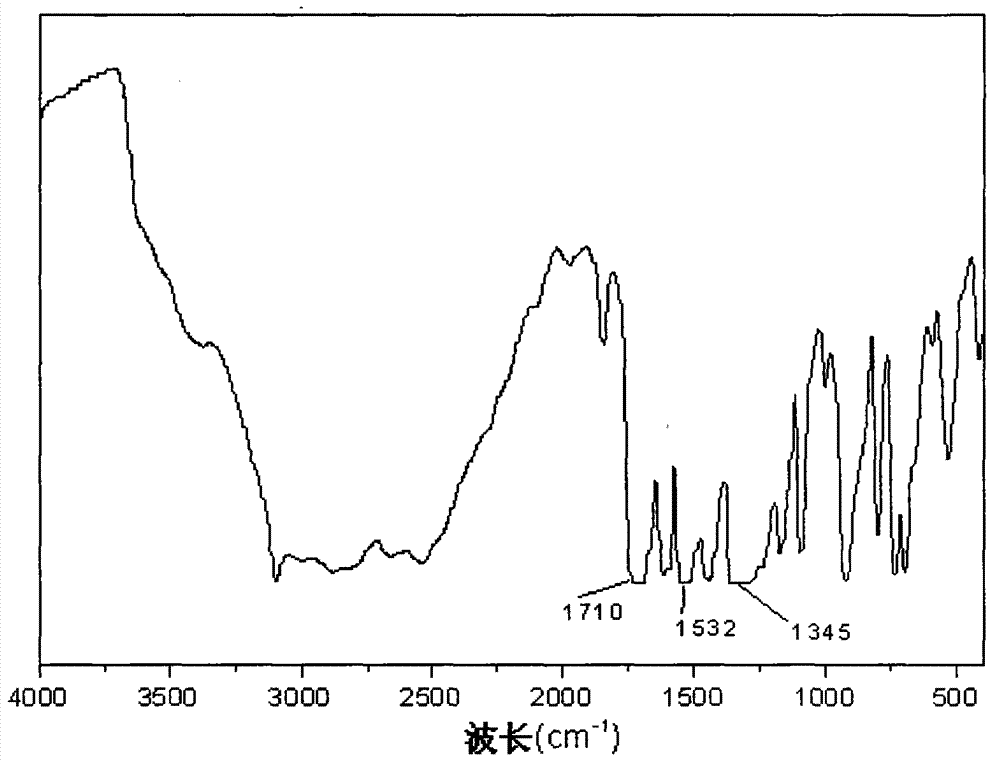
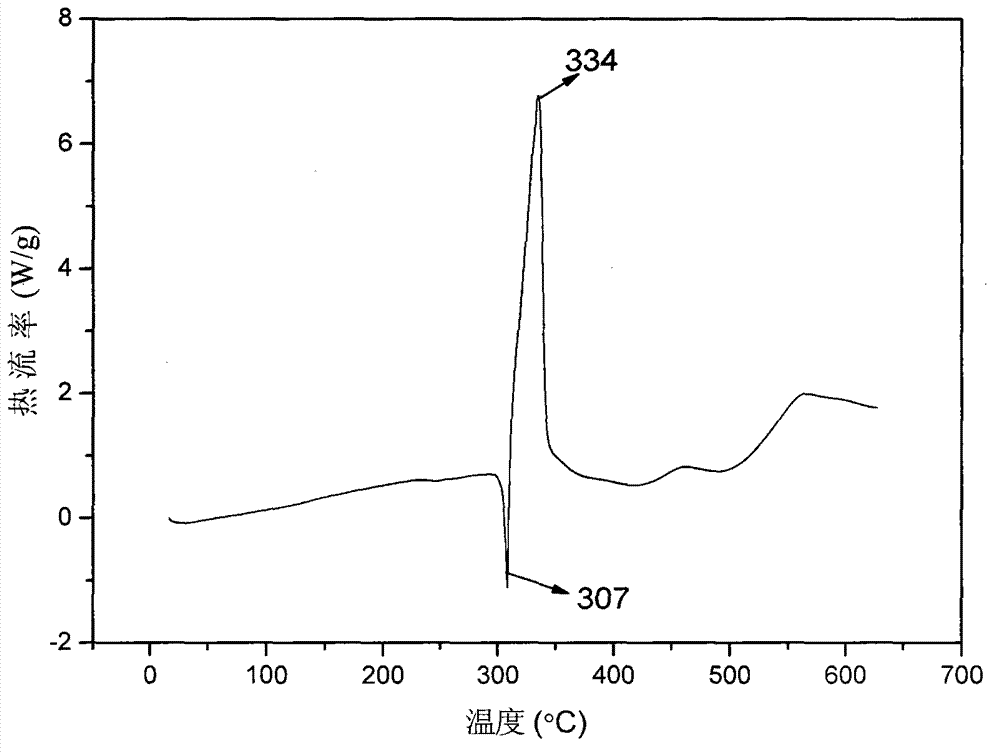
Synthetic method for 4,4',6,6'-tetranitro-2,2'-diphenic acid
A technology of biphenyl dicarboxylic acid and tetranitro, which is applied in 4 fields, can solve the problems of small quantity and non-single product, and achieve the effect of low cost and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Add 11.28 g of 2,2'-biphenyldicarboxylic acid (prepared by yourself according to the method disclosed in the Journal of Dalian Institute of Technology, 1988, Volume 27 Supplement: 73-78) in a 500 mL three-necked flask. Under the condition of 15°C, stir continuously and slowly add 75mL of concentrated sulfuric acid (Sinopharm Chemical Reagent Co., Ltd., product number: 73108460), the color of the solution gradually turns orange during the acid addition process, and add all concentrated sulfuric acid After (approximately 2 hours), continue to stir for 10min;
[0028] 2. Measure 25mL of fuming nitric acid (Sinopharm Chemical Reagent Co., Ltd., article number: 10014617) and slowly add it dropwise to the above mixture. After dripping (approximately 1.5 hours), there was a small amount of brown gas in the bottle. Continue to stir for 30 minutes under ice bath conditions, then slowly raise the temperature to 90°C (heating time is 70 minutes), and keep it for 5 hours;
[00...
Embodiment 2
[0034] 1. Add 4.5g of 2,2'biphenyl dicarboxylic acid to a 250mL three-neck flask, and stir continuously under ice bath conditions and slowly add 30mL of concentrated sulfuric acid. After all the concentrated sulfuric acid is dropped, stir for 10min;
[0035] 2. Measure 10mL of fuming nitric acid and slowly add it to the above mixture. After the fuming nitric acid is dripped, stir for 30 minutes in an ice bath, then raise the temperature to 70°C and keep it for 5 hours;
[0036] 3. After the reaction is completed, cool down to room temperature, slowly add the mixture into a beaker with crushed ice, stir, and let stand;
[0037] 4. Suction filter the above mixture, wash with water three times, and dry to obtain 6.08 g of 4,4',6,6'-tetranitro-2,2'-biphenyldicarboxylic acid product.
[0038] The yield was calculated according to the method in Example 1, and the yield was 86%.
Embodiment 3
[0040] 1. Add 4.5g of 2,2′-biphenyldicarboxylic acid to a 250mL three-neck flask, and under ice bath conditions, continuously stir and slowly add 30mL of concentrated sulfuric acid, after all the concentrated sulfuric acid is dropped, stir for 10min;
[0041] 2. Measure 30mL of concentrated nitric acid and slowly add it to the above mixture. After the fuming nitric acid is dripped, stir for 30min in an ice bath, then raise the temperature to 90°C and keep it for 1h;
[0042] 3. After the reaction is completed, cool down to room temperature, slowly add the mixture into a beaker with crushed ice, stir, and let stand;
[0043] 4. Suction filter the above mixture, wash with water three times, and dry to obtain 5.77 g of 4,4',6,6'-tetranitro-2,2'-biphenyldicarboxylic acid product.
[0044] The yield was calculated according to the method in Example 1, and the yield was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com