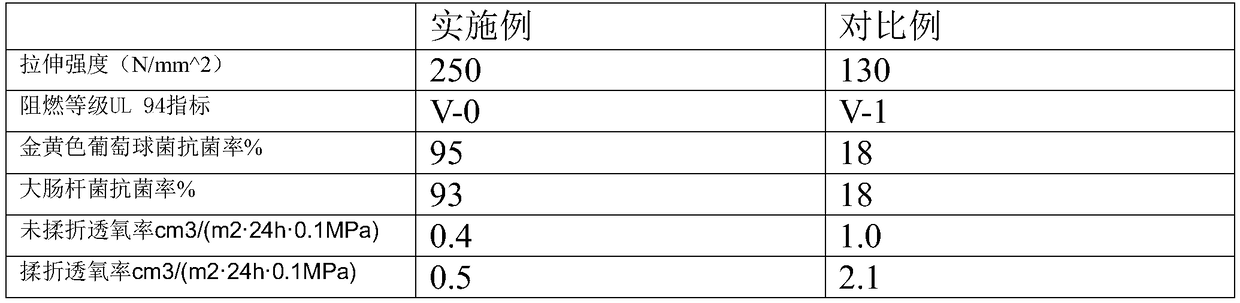
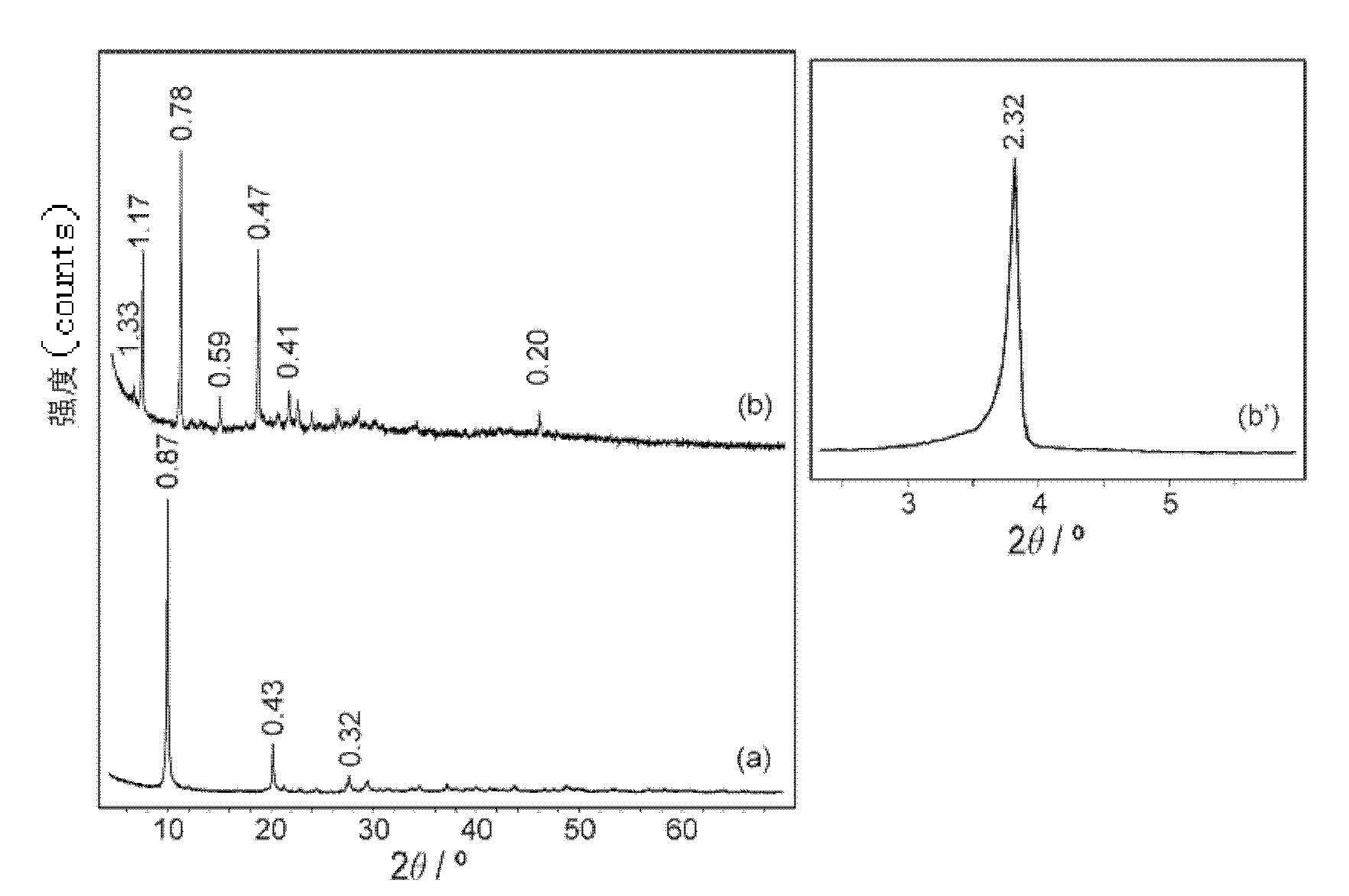
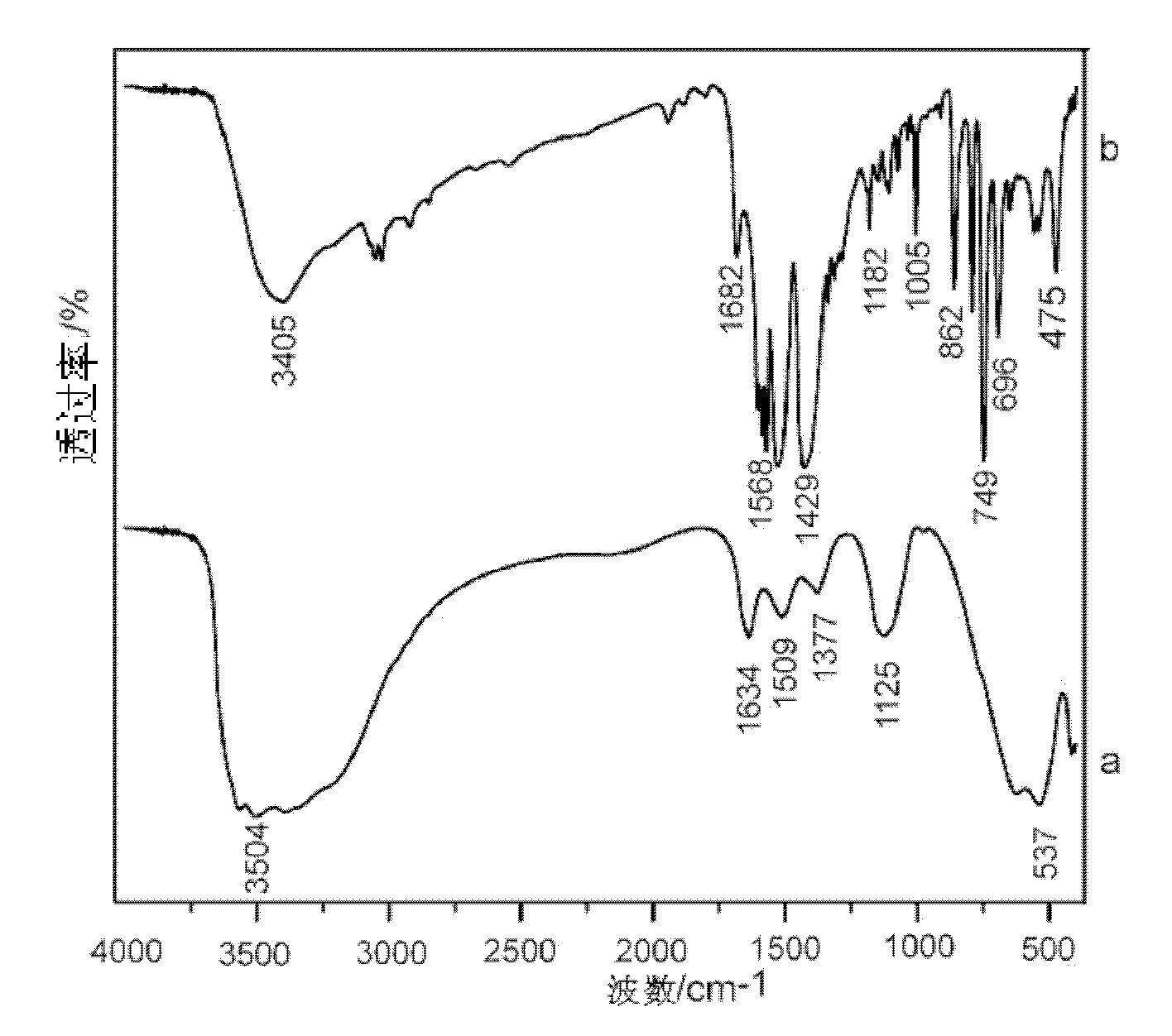
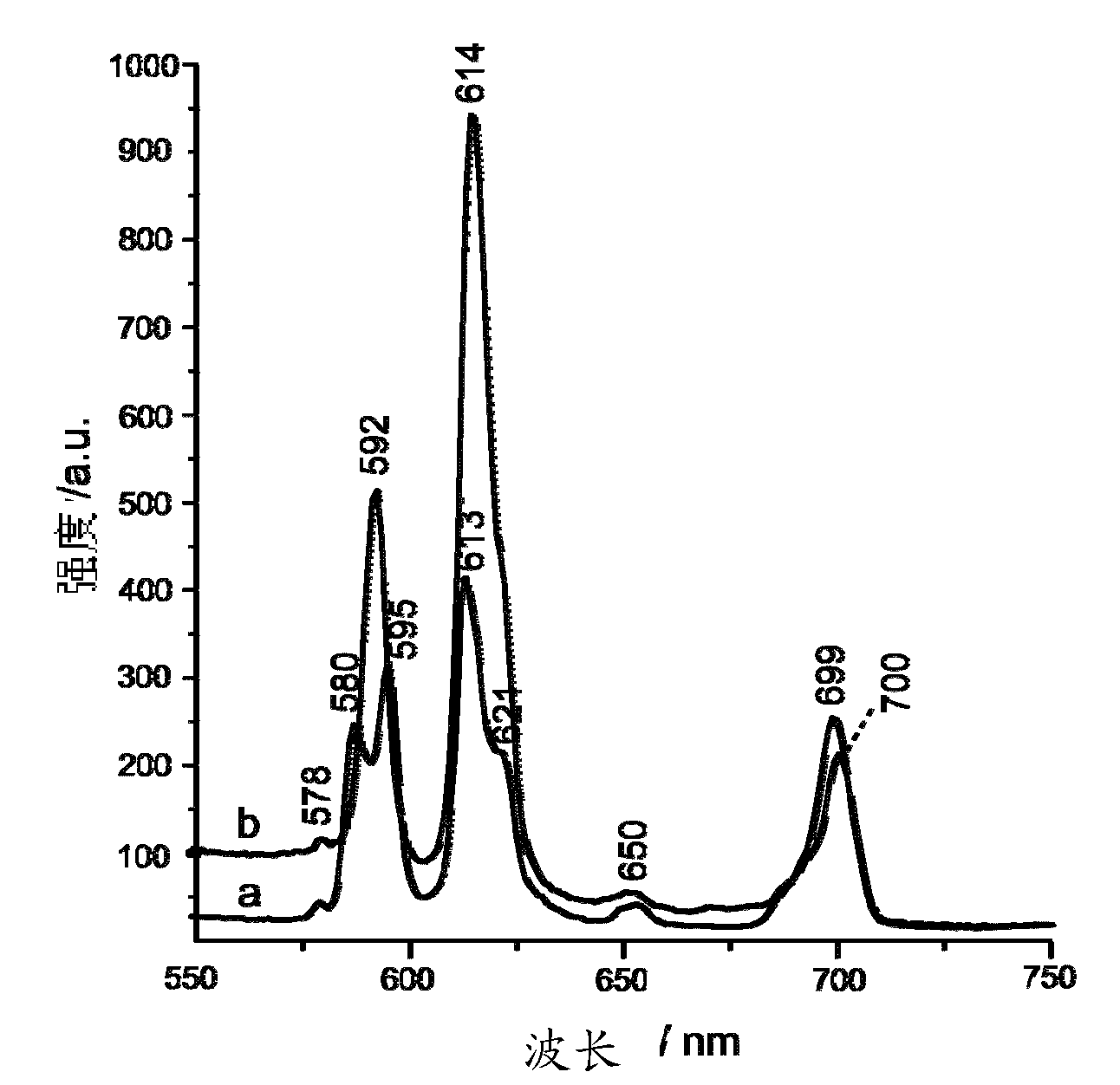
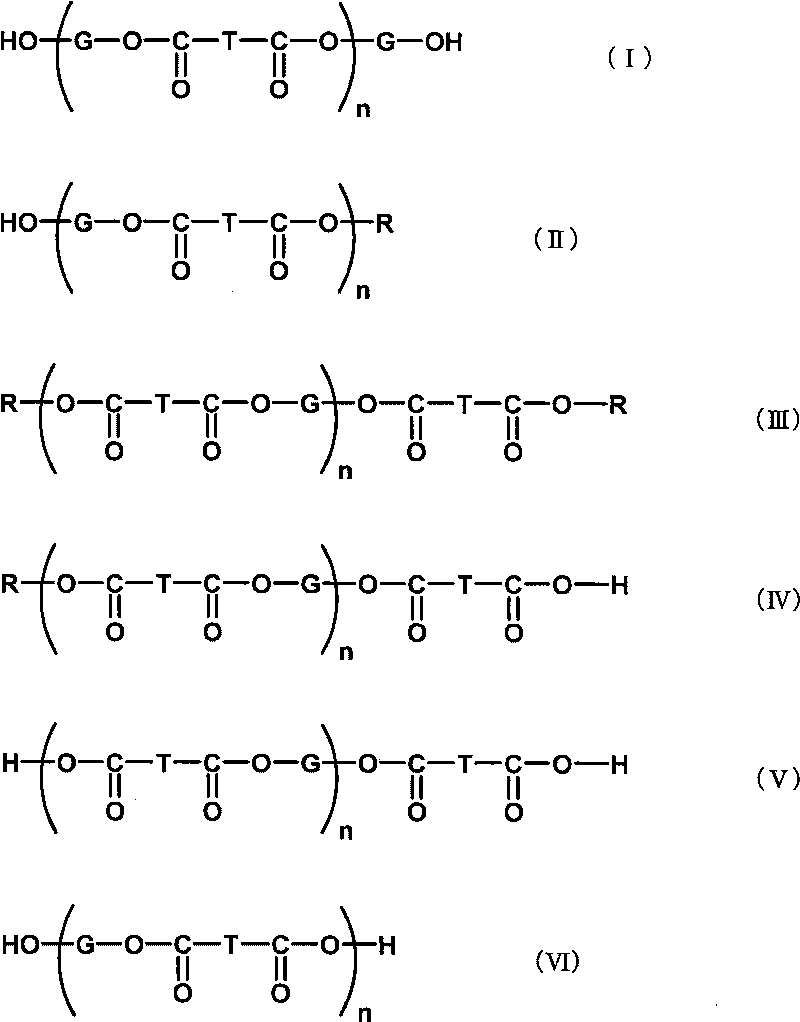Patents
Literature
30 results about "Diphenic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
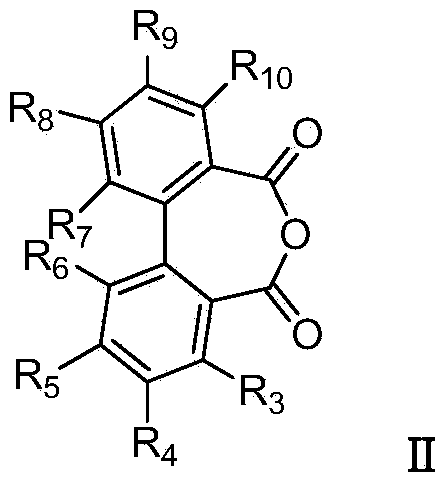
Additive for cellulose ester resin, cellulose ester resin composition using same, and film
ActiveCN101735477AResistance to exudationGood optical performancePolarising elementsNon-linear opticsCelluloseMoisture penetration
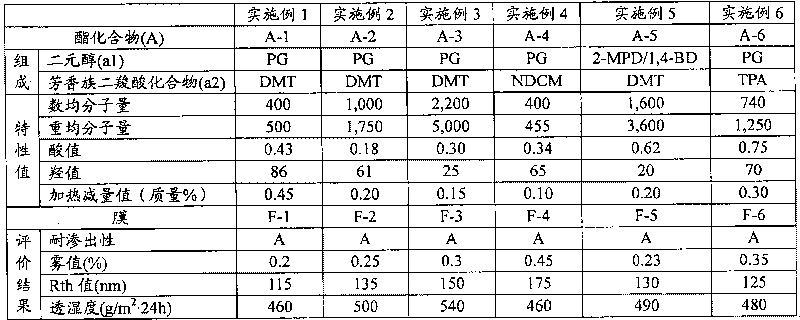
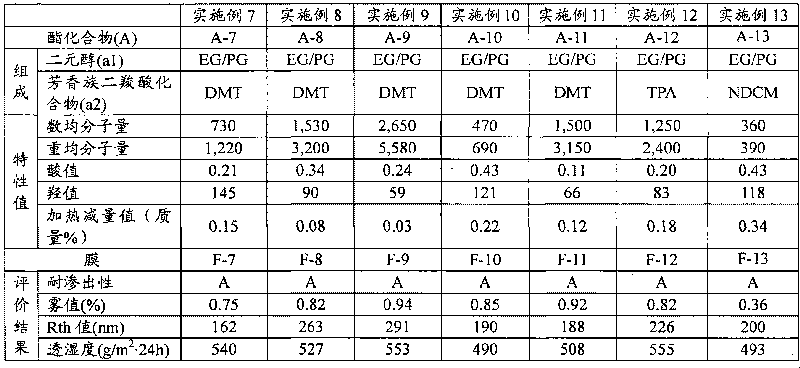
The present invention provides an additive for cellulose ester resin, which is obtained by esterification between dibasic alcohol (s1) and aromatic dicarboxylic acid compound (a2) and has a number-average molecular weight in a range of 300-3,000. The aromatic dicarboxylic acid compound (a2) is more than one component selected from terephthalic acid, terephthalic dialkyl ester, naphthalenedicarboxylic acid, naphthalenedicarboxylic dialkyl ester, diphenic acid and diphenic dialky ester. The additive for cellulose ester resin can make an optical film to have excellent optical performance and moisture-penetration resistance. Furthermore the optical film with excellent moisture-penetration resistance in high temperature and high moisture is provided.
Owner:DIC CORP
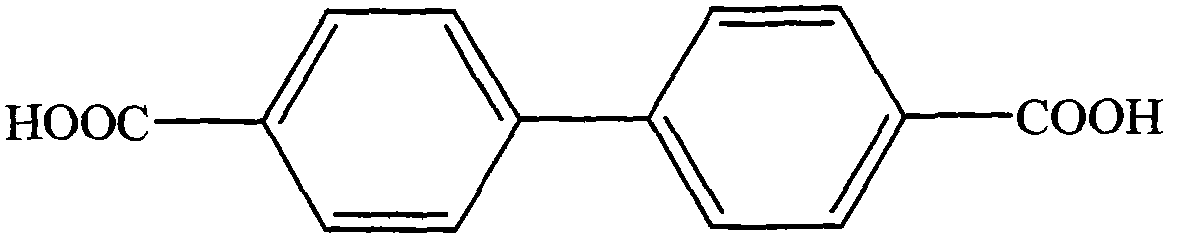
Method for synthesizing 4,4'-diphenic acid
InactiveCN102701964AThe synthesis steps are simpleSynthetic steps are environmentally friendlyOrganic compound preparationCarboxylic compound preparationPurification methodsSteam pressure
The invention provides a method for synthesizing 4,4'-diphenic acid. In the method, high-boiling-point polyethylene glycol 400 is used as a solvent, cuprous iodide (CuI) is used as a catalyst, and 4,4'-diphenic acid is obtained in the condition of presence of diethylenediamine and potassium hydroxide (KOH) by a reduction coupling new method. The synthesis method has the advantages that the method for synthesizing 4,4'-diphenic acid is simple, the synthesizing condition requirements are not high, the operation is easy, the production cost is low, the yield is very high, the purity is also very high and the repeatability is good, so that the method is suitable for the requirements of large-scale industrialized production; the solvent polyethylene glycol 400 used by the reaction system has high steam pressure, does not pollute the environment and is environment-friendly; and the purification method is simple, column separation purification is not needed, and purification can be carried out just by a common recrystallization method.
Owner:JIANGXI NORMAL UNIV
Polyester resin for anti-yellowing HAA (N,N,N',N'-tetra(Beta-hydroxyethyl)adipamide) system powder paint, preparation method and application
ActiveCN109054008AImprove high temperature resistanceImprove anti-yellowing effectPowdery paintsPolyester coatingsDiphenic acidPyromellitic dianhydride
The invention belongs to the technical field of resin production, and particularly relates to a polyester resin for an anti-yellowing HAA (N,N,N',N'-tetra(Beta-hydroxyethyl)adipamide) system and further relates to a preparation method and application of the polyester resin. The anti-yellowing HAA system provided by the invention is polymerized by using pyromellitic dianhydride, 1,6-hexanediamine,isophthalic acid, phloroglucinol formic acid, ethanetricarboxylic acid monoester, 2,5-dihydroxy hexane, neopentyl glycol and 2,2'-diphenic acid. Because a polyimide chain is carried in the molecular chain of the polyester resin obtained by the materials and a specific process, the high temperature-resistant and anti-yellowing properties are excellent; moreover, because the materials containing phenolic hydroxyl groups, such as phloroglucinol formic acid and ethanetricarboxylic acid monoester, are used for participating in reaction and the phenolic functional group with high anti-yellowing capability is contained in the polyester chain, the polyester product has excellent high temperature-resistant and anti-yellowing properties, and is ultimately used in the powder paint of the HAA system,so that a coated panel with excellent anti-yellowing property can be obtained.
Owner:HUANGSHAN HUIZHOU KANGJIA CHEM CO LTD
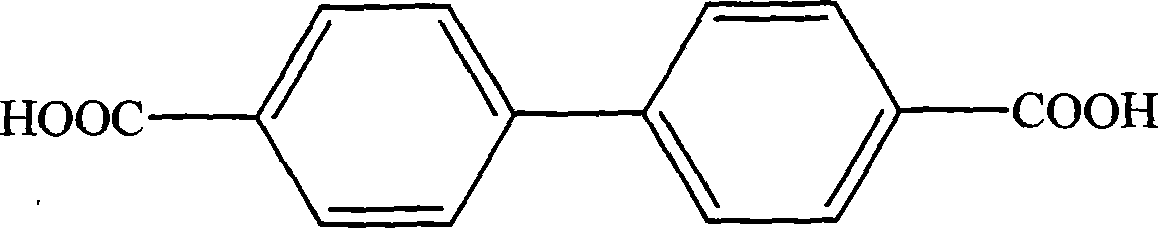
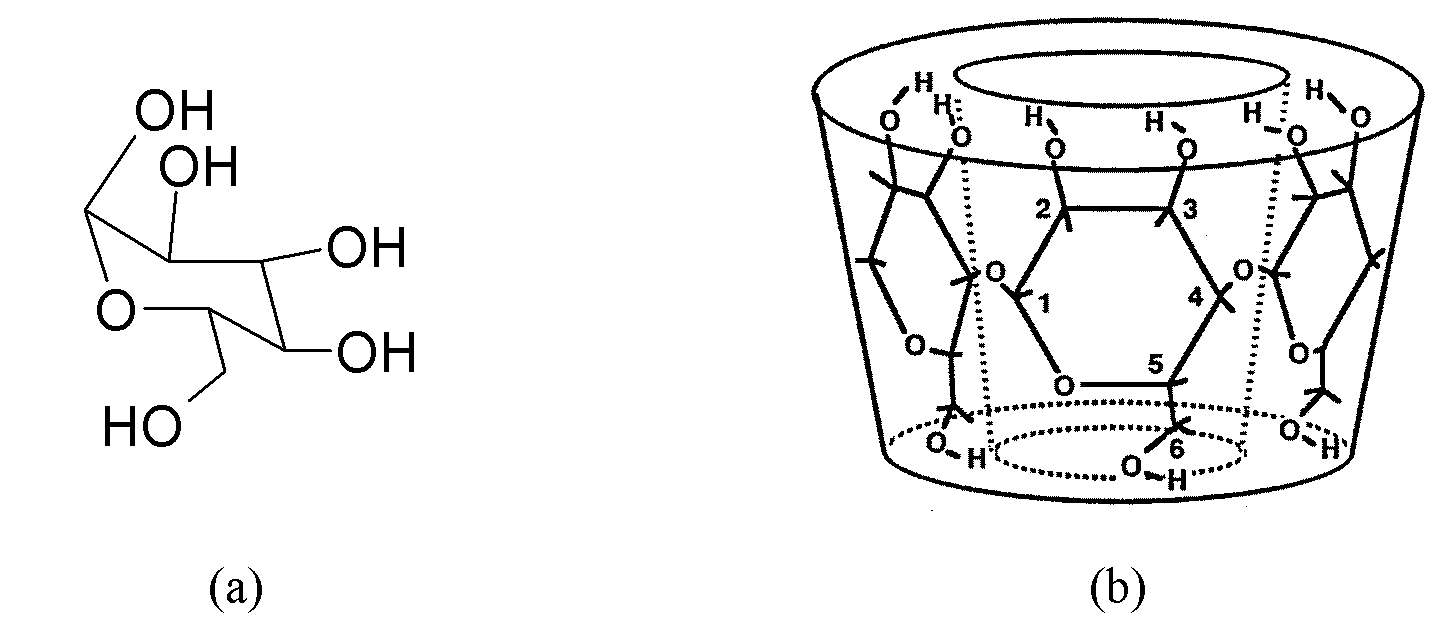
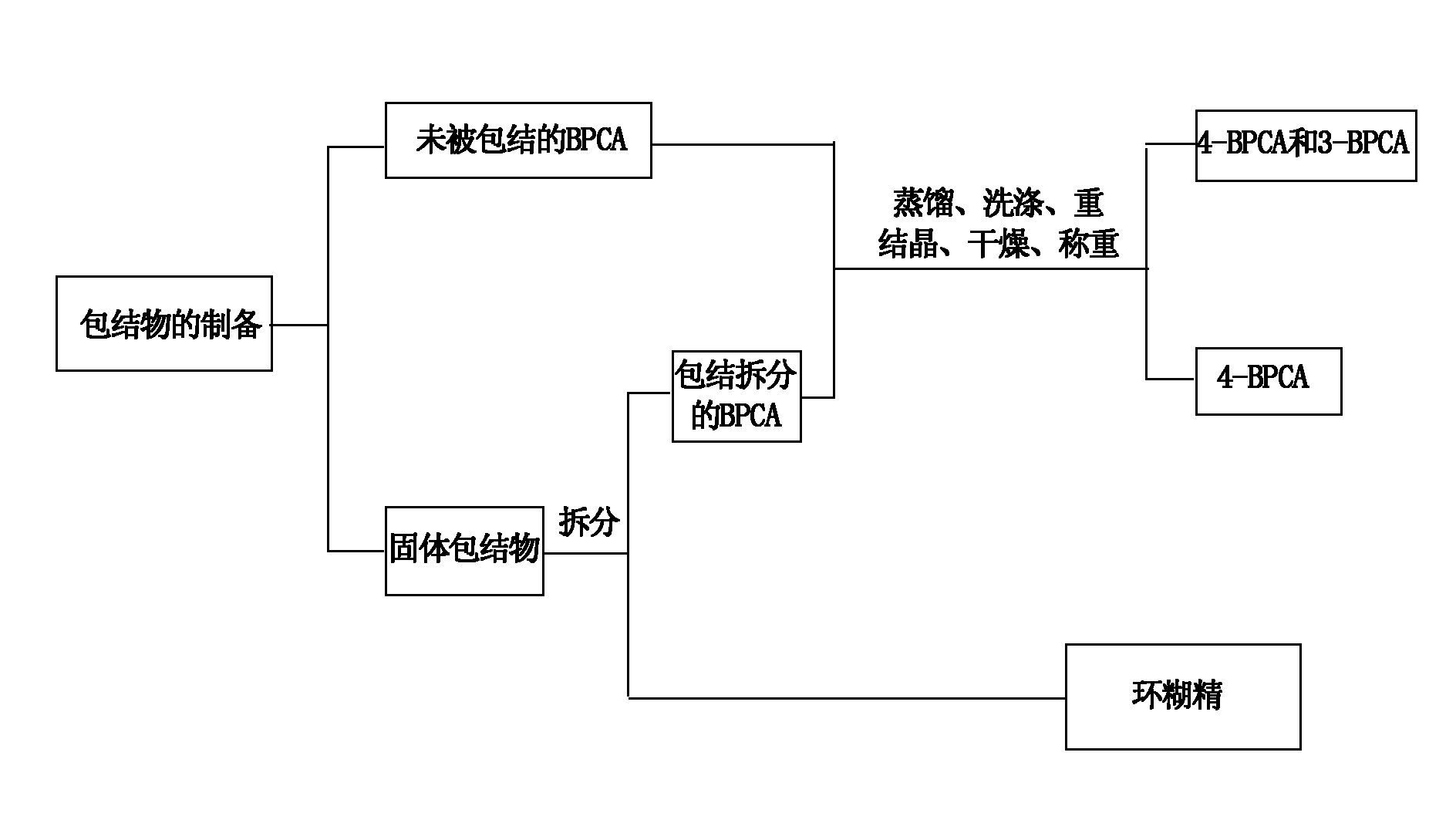
Inclusion separating method and application of isomerides of 3-diphenic acid and 4-diphenic acid
InactiveCN101891609AExcellent separation functionEasy to operateCarboxylic compound separation/purificationDiphenic acidBeta-Cyclodextrins
The invention relates to an inclusion separating method and application of isomerides of 3-diphenic acid and 4-diphenic acid. In the method, a mixture of 3-diphenic acid and 4-diphenic acid is separated by beta-cyclodextrin inclusion. The method comprises the following steps of: preparing an inclusion compound, (2) resolving the inclusion compound, and (3) processing the product. According to the difference between molecular structures of the 4-diphenic acid and the 3-diphenic acid, a host molecule chooses molecules which are complementary to the host molecule for inclusion and separates the molecules out in the form of crystals so as to achieve the separation purpose. On the basis of the research on the influences of material proportioning, heating temperature, inclusion time and other factors on the inclusion separating result, the optimal separating conditions for the preparation method and preparation conditions of the inclusion compound are found by orthogonal tests. The purity of the 4-diphenic acid is up to over 99%.
Owner:TIANJIN UNIV
Polyamide resin compsns.
InactiveCN1343742AImprove flame retardant performanceGood chemical resistancePolyamideCarboxylic acid
A polyamide resin composition is provided, which contains 100 parts by weight of a polyamide resin (A) that has dicarboxylic acid units (a) containing from 60 to 100 mol% of terephthalic acid units and diamine units (b) containing from 60 to 100 mol% of C6-18 aliphatic alkylenediamine units, and from 1 to 100 parts by weight of a polybromostyrene (B), in which from 0.5 to 100% by weight of the polybromostyrene (B) is a polybromostyrene having an epoxy group. The polyamide resin composition has good flame retardancy, good chemical resistance, good surface appearance and good blistering resistance.
Owner:KURARAY CO LTD
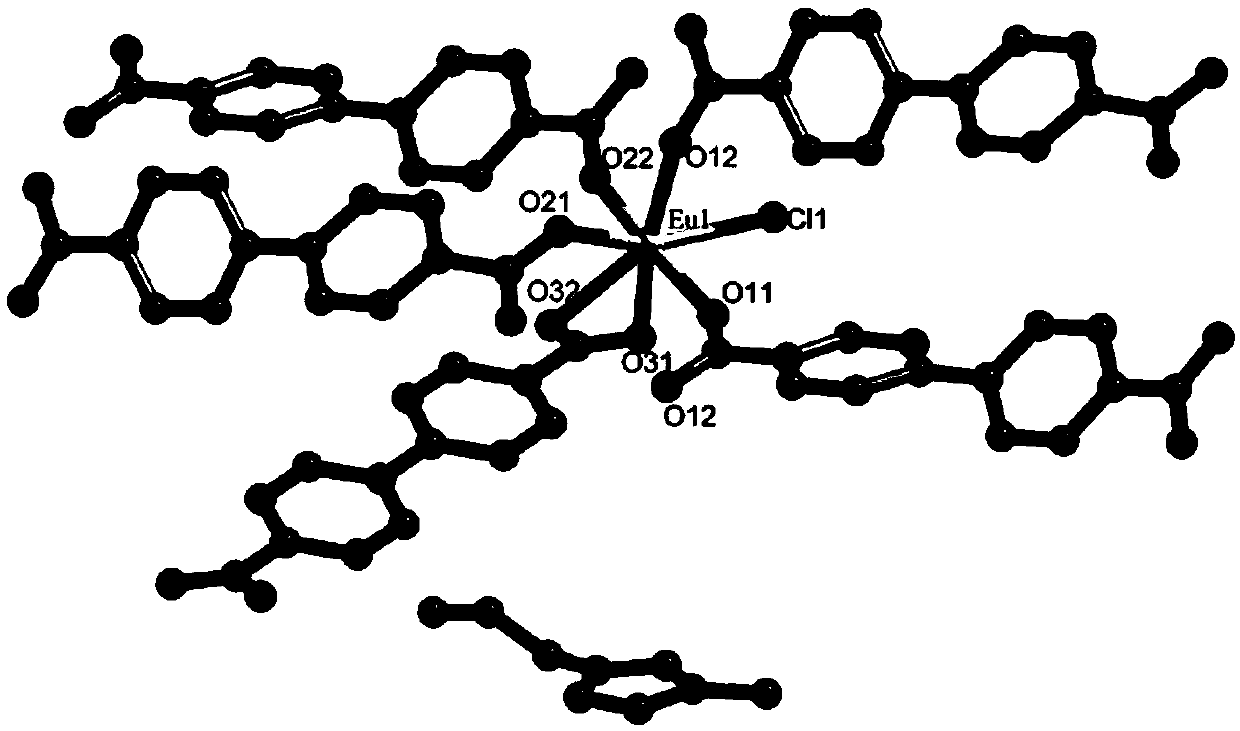
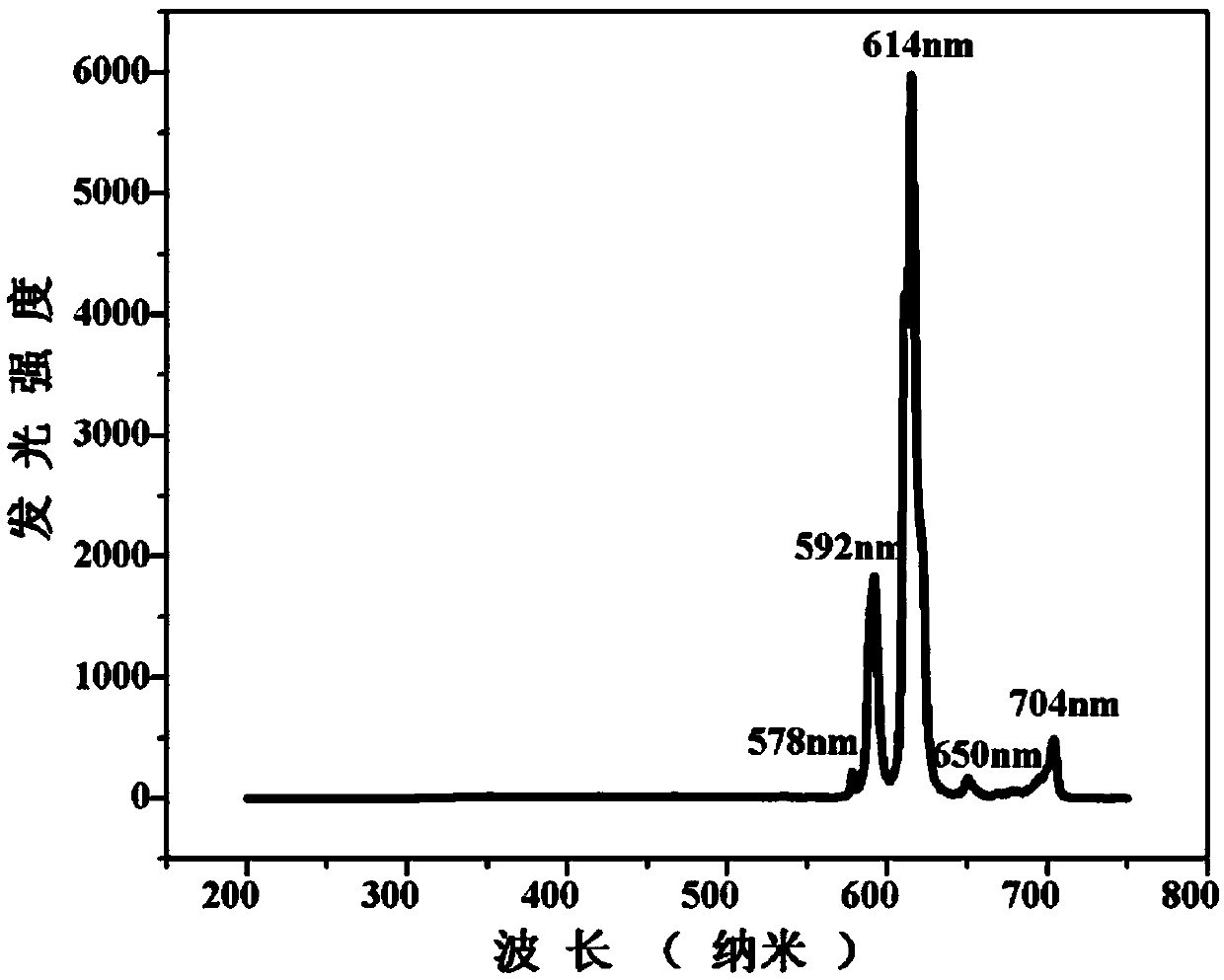
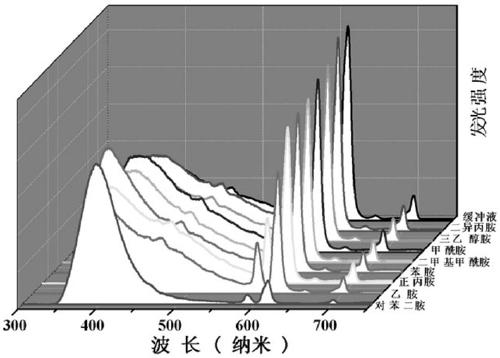
Europium fluorescent probe and test paper based on diphenic acid and application thereof in the detection of p-phenylenediamine
ActiveCN107057685ABright redObviously Eu
<sup>3+</sup>
characteristic emission peakGroup 3/13 organic compounds without C-metal linkagesFluorescence/phosphorescenceDiphenic acidCrystal cell
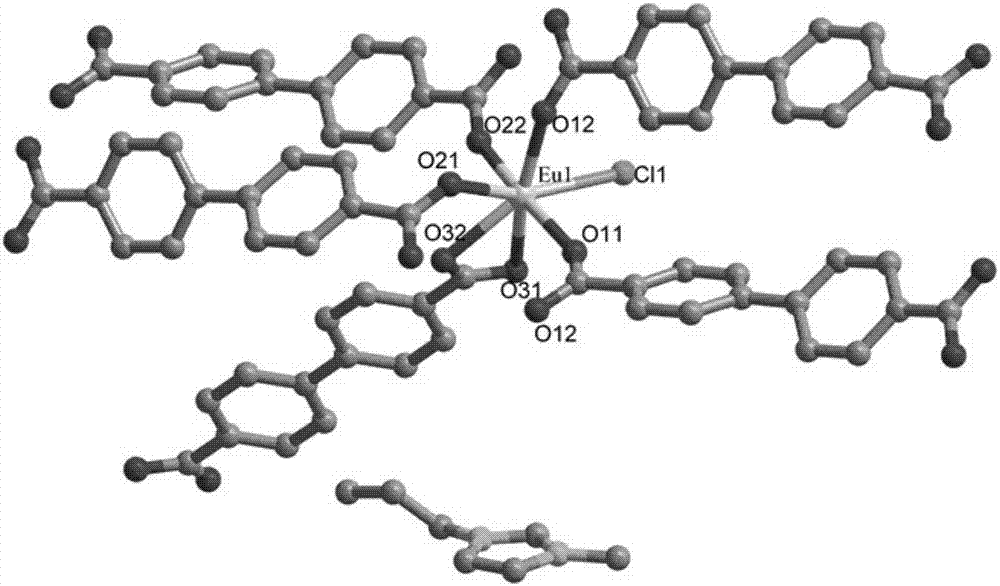
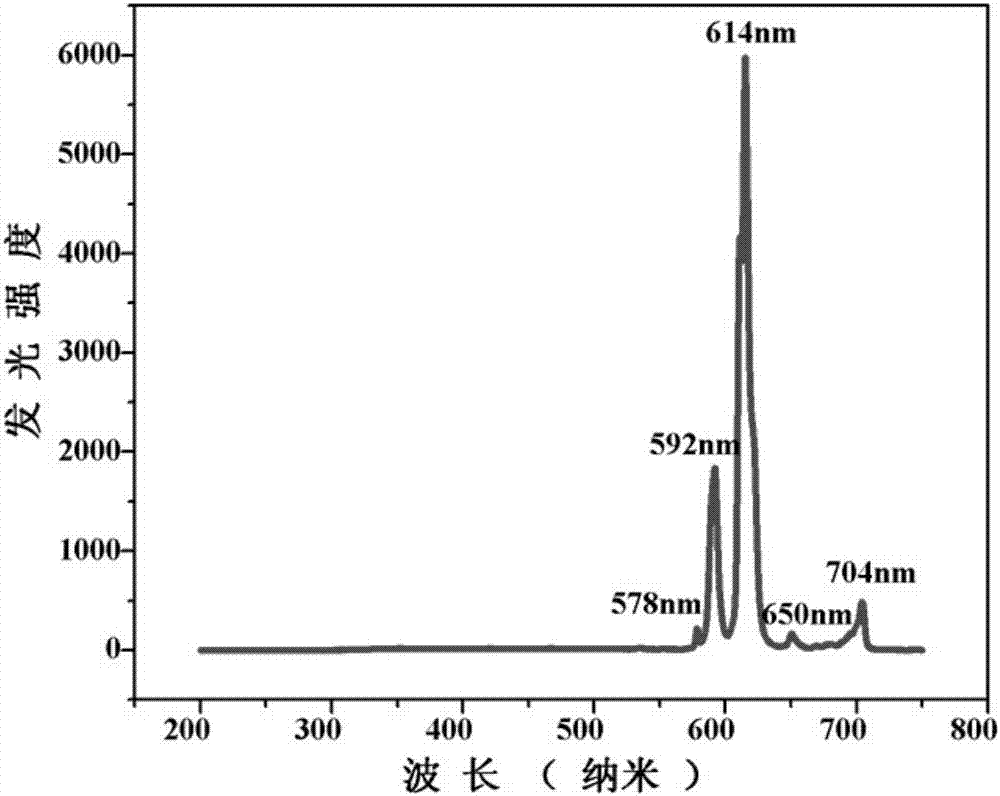
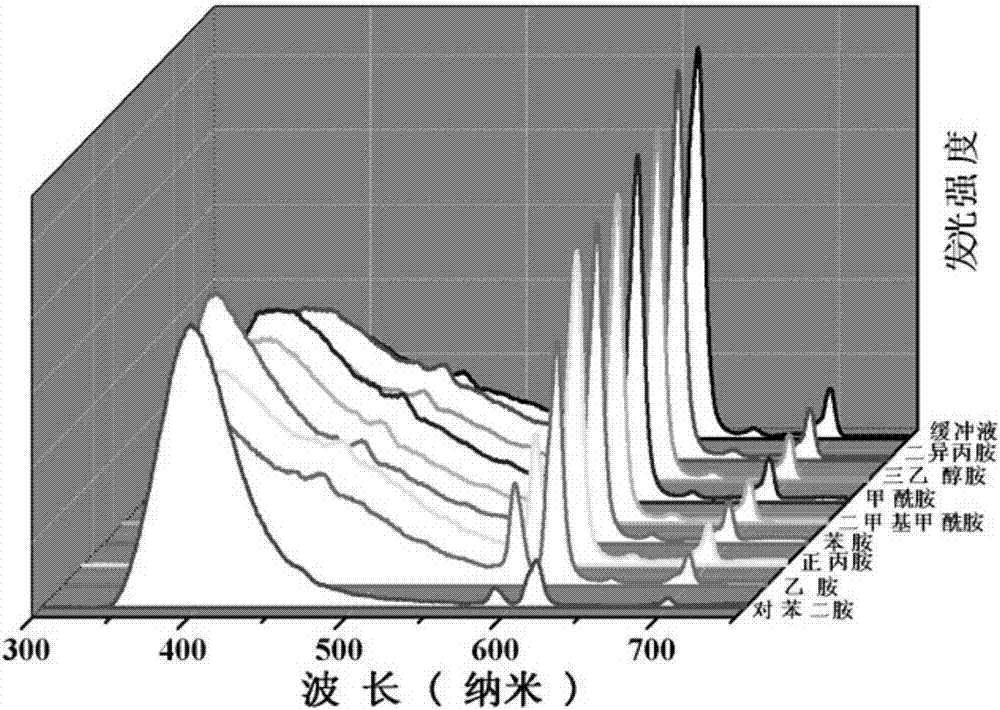
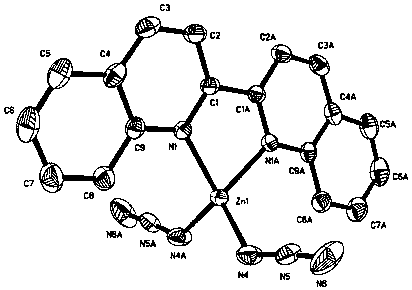
The invention discloses an Europium fluorescent probe and test paper based on diphenic acid and application thereof in the detection of p-phenylenediamine, the structural unit of the fluorescent probe is [PMI]2[Eu2(BPDC)3C12], wherein the PMI represents 1-propyl-3-methylimidazole monovalent cation, BPDC represents diphenic acid radical that loses protons on two carboxyl groups; the fluorescent probe belongs to a monoclinic crystal system and the space group is P21 / c. the crystal cell parameters are as follows: alpha is equal to gamma, which is equal to 90 degree, belta is equal to 96.6830(10) degree; and the crystal cell volume of z is equal to 4. The fluorescent probe of the invention has excellent red fluorescence properties and shows bright red under the irradiation of an ultraviolet lamp, the p-phenylenediamine in the aqueous phase has a significant quenching effect on the red fluorescence of the fluorescent probe and test paper, so the fluorescent probe and test paper can be used for detecting p-phenylenediamine with high sensitivity, high selectivity and anti-interference, the fluorescent probe and test paper are disposable (similar to normal pH test paper).
Owner:SHAANXI NORMAL UNIV
Method for detecting 1,2-benzenedicarboxylic acid-dialkyl ester plasticizer in plastic through gas chromatography-mass spectrometry
The invention relates to a method for detecting a 1,2-benzenedicarboxylic acid-dialkyl ester plasticizer in plastic through gas chromatography-mass spectrometry. The method comprises the steps that 1, a standard solution is prepared; 2, instruments and measurement work conditions are optimized; 3, a sample to be detected is prepared, purified and extracted; 4, a standard curve is built; 5, the detection limit, the lower quantitation limit, the precision and the recovery rate of the method are determined; 6, the sample to be detected is determined according to the determination method built in the step 1 to the step 5. According to the method, the detection efficiency and the detection accuracy are improved.
Owner:国家再生有色金属橡塑材料质量监督检验中心(安徽)
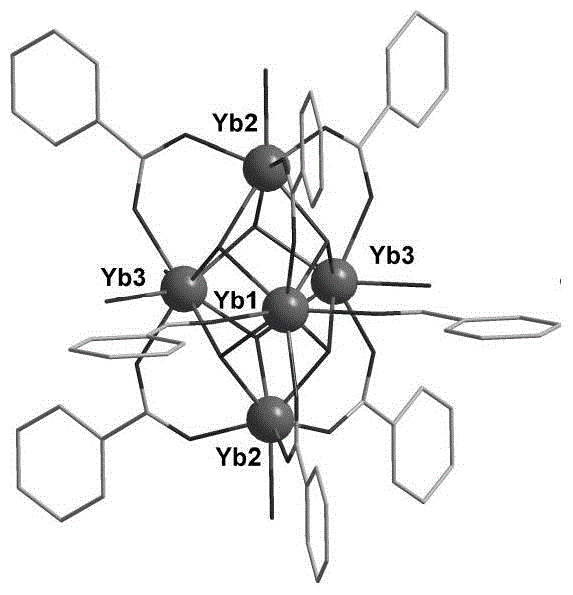
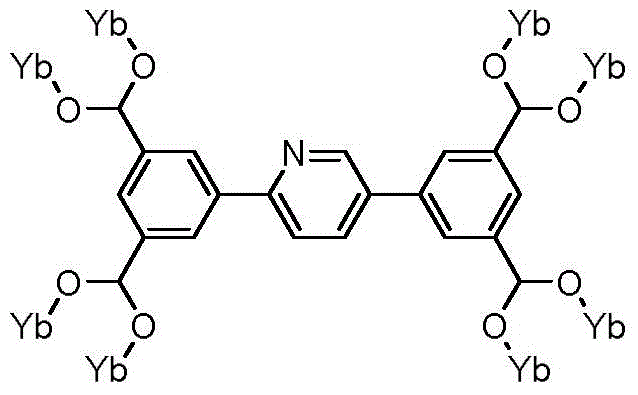
Pentanuclear-ytterbium-cluster-molecule-structure-unit-conitaning metal organic framework, and preparation method and application thereof
ActiveCN105732487AImprove catalytic performanceThe synthesis method is simpleOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsN dimethylformamideCycloaddition
The invention relates to a pentanuclear-ytterbium-cluster-molecule-structure-unit-conitaning metal organic framework, and a preparation method and application thereof. The technical scheme is as follows: adding Yb(NO3)3, a 5,5'-(pyridyl-2,5-disubstituted)-1,3-phthalic acid, a solvent N,N-dimethylformamide and water into a high-temperature-resistant screw glass bottle, and stirring at normal temperature; sealing the container, putting into a drying oven, and heating at 105 DEG C for 4 days; slowly cooling to room temperature to obtain colorless transparent massive crystals; and washing with N,N-dimethylformamide, filtering, and drying to obtain the target product. The pentanuclear-ytterbium-cluster-molecule-structure-unit-conitaning metal organic framework has favorable size selectivity and catalytic property for carbon dioxide cycloaddition reaction of epoxides.
Owner:LIAONING UNIVERSITY
Process for preparing 4'-trifluoro methyl-2-diphenyl formic acid
InactiveCN1944386ARaw materials are cheap and easy to getLow pricePreparation from nitrilesGrignard reagentDiphenic acid
The present invention discloses process of preparing 4'-trifluoro methyl-2-diphenic acid. The process includes the following steps: 1. the reflux reaction of p-chloro trifluorotoluene and magnesium chip in anhydrous tetrahydrofuran under nitrogen protection to obtain Grignard reagent; 2. mixing o-chloro phenyl nitrile, 1, 2-bis(diphenyl phosphine) ethane nickel chloride and tetrahydrofuran via stirring; dropping the Grignard reagent; filtering and eliminating solvent to recover tetrahydrofuran; adding toluene and water solution of ammonium chloride; and oil-water separating to obtain toluene solution of 4'- trifluoro methyl-2-cyan diphenyl; and 3. heating the toluene solution in alkaline water solution to hydrolyze; oil-water separation to obtain water solution of 4'-trifluoro methyl-2-diphenic sodium and further treatment to obtain 4'-trifluoro methyl-2-diphenic acid. The process has high yield, low cost and other advantages, and is suitable for industrial production.
Owner:ZHEJIANG UNIV
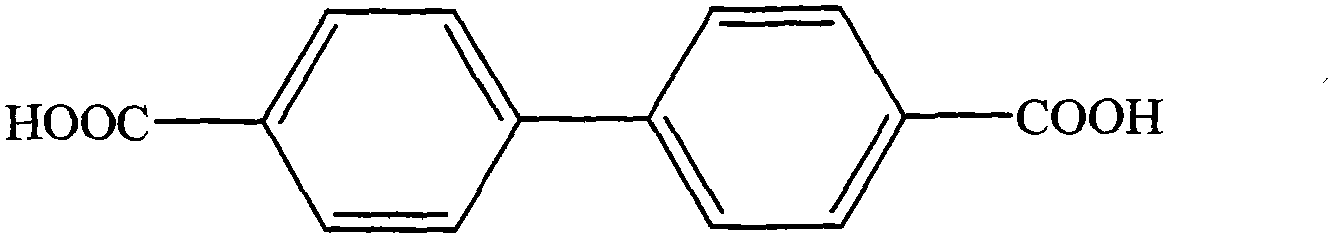
Synthesis method for 4,4'-biphenyldicarboxylic acid
ActiveCN103483186AAtom economy is highHigh yieldOrganic compound preparationCarboxylic preparation by oxidationChemical synthesisSynthesis methods
The invention relates to the field of chemical synthesis, and in particular to a synthesis method for 4,4'-biphenyldicarboxylic acid. The synthesis method for 4,4'-biphenyldicarboxylic acid is characterized by comprising the following reaction steps of: step 1, reacting 4-diphenic acid, oxalyl chloride and aluminium trichloride in a molar ratio of 1: (1.1-1.8): (1.2-2.0) to obtain 4,4'-dicarboxylbibenzil, wherein the weight ratio of 4-diphenic acid to a solvent is 1: (3 to 10); and step 2, reacting 4,4'-dicarboxylbibenzil, an oxidant and potassium hydroxide in a molar ratio of 1: (1.2-2.0):(1.4-2.2), and then carrying out recrystallization by absolute methanol to obtain 4,4'-biphenyldicarboxylic acid, wherein the weight ratio of 4,4'-dicarboxylbibenzil to an organic solvent is 1: (3-10). Compared with the prior, the synthesis method for 4,4'-biphenyldicarboxylic acid disclosed by the invention has the beneficial effects of being reasonable and practicable in process flow, high in yield which is up to move than 93%, moderate in process reaction conditions, low in requirements on equipment, and suitable for industrialized production.
Owner:SINOSTEEL ANSHAN RES INST OF THERMO ENERGY CO LTD
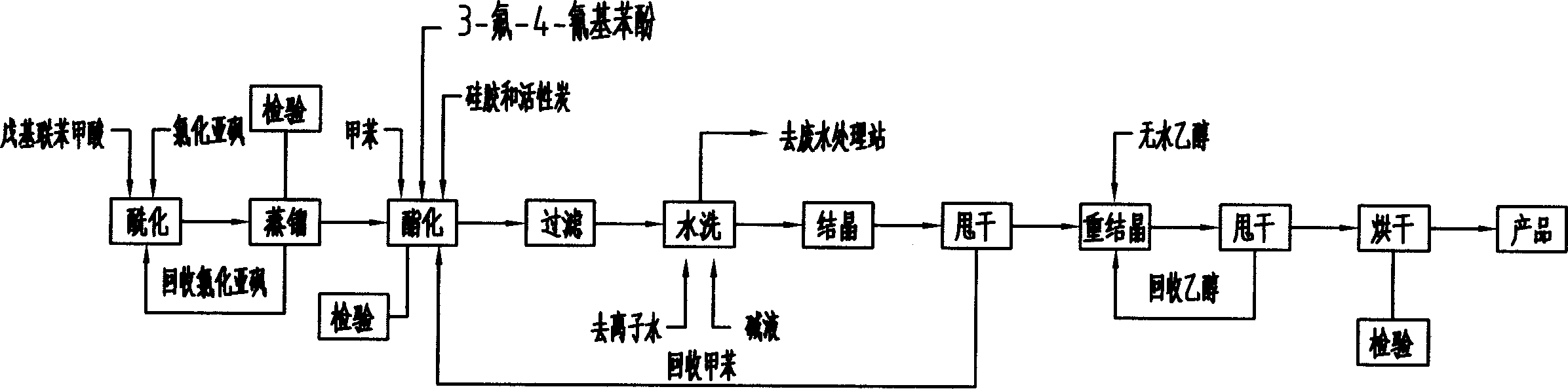
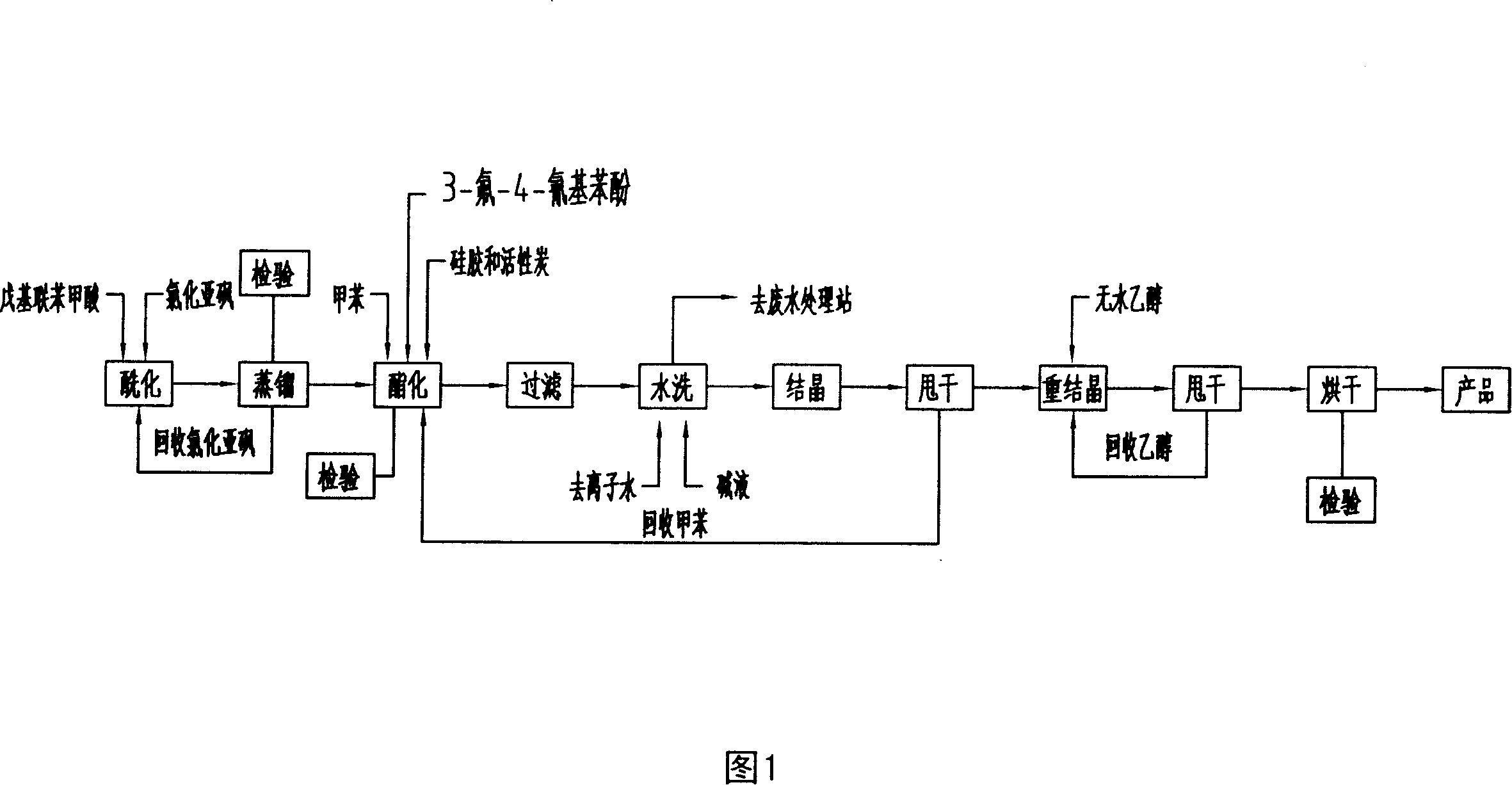
4-nor-amyl diphenic acid-3-fluor-4-cyan phenol ester production process
ActiveCN1865239AThe reaction conditions are reasonable and feasibleReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationSteam pressureDistillation
This invention relates to a method for producing 4-amyl-biphenylcarboxylic acid-3-fluoro-4-cyanophenol ester, comprising: amyl-biphenylcarboxylic acid, thionyl chloride, 3-fluoro-4-cyanophenol, toluene, sodium-hydroxide, and anhydrous ethyl alcohol as raw material, with mole ration as: amyl-biphenylcarboxylic acid 1.05, thionyl chloride 2.65, 3-fluoro-4-cyanophenol 1, toluene 3, sodium-hydroxide 0.1, and anhydrous ethyl alcohol 4.0. The process comprises two steps: (1) use amyl-biphenylcarboxylic acid and thionyl chloride as raw material for acylation reaction and synthesize amyl-bibenzoyl chloride by normal pressure and decreasing pressure distillation; (2) dropwise add amyl-bibenzoyl chloride and 3-fluoro-4-cyanophenol with toluene as solvent for esterification, and produce the target product 4-amyl-biphenylcarboxylic acid-3-fluoro-4-cyanophenol ester by water washing, alkaline washing, crystallization and recrystallization, wherein the conditions: acylation reaction temperature 160Deg C, reflux time 6h, normal distillation temperature 172-176Deg C, pressure decreasing temperature 118-121Deg C, vacuum 50mmHg, the discharging condition is to collect the 3Deg C range product after temperature and pressure stabilized, the temperature of adding 3-fluoro-4-cyanophenol with agitation for acylation reaction is 100Deg C, keep the temperature between 90 and 100Deg C when dropwise adding amyl-bibenzoyl chloride for 3h, reflux with agitation for 6h, heating steam pressure no more than 0.2MPa, autoclave inside temperature kept at 120+2Deg C, crystallization temperature -15Deg C and kept at -15+-2Deg C for 1h for agitation, recrystallization temperature -15Deg Cand kept at -15+-2Deg C for 1h for agitation.
Owner:江苏和纯化学工业有限公司
2, 2'-biquinoline azide-zinc metal complex and preparation method and application thereof
InactiveCN108017661AOrganic chemistry methodsGroup 2/12 organic compounds without C-metal linkagesQuinolineDiphenic acid
The invention discloses a 2, 2'-biquinoline azide-zinc metal complex and a preparation method and application thereof. The preparation method utilizes a solvothermal synthesis method and comprises that reactants such as zinc nitrate hexahydrate, sodium azide and 2, 2'-biquinoline undergo a reaction according to a mole ratio of 1: 2: 1 in a solvent which is a mixed solution of an appropriate amountof 2, 2'-diphenic acid, methanol and water at a temperature of 130-150 DEG C, the reaction system is kept at the temperature for 3 days and then is cooled to the room temperature, the reaction product is filtered, then the filter cake is washed through diethyl ether so that the 2, 2'-biquinoline azide-zinc metal complex monocrystal C18H12N8Zn is obtained. The complex has a single-molecule structure. Through a hydrogen bond and pai...pai aromatic ring stacking, the three-dimensional supramolecular structure is obtained. Through ultraviolet excitation, strong fluorescence is produced at 418 nm.The 2, 2'-biquinoline azide-zinc metal complex is a novel fluorescent material and can be used for preparing fluorescent emitting devices.
Owner:ANQING NORMAL UNIV
Method for synthesizing 4,4'-diphenic acid
InactiveCN102701964BThe synthesis steps are simpleSynthetic steps are environmentally friendlyOrganic compound preparationCarboxylic compound preparationSteam pressurePurification methods
Owner:JIANGXI NORMAL UNIV
Synthesis method for 4,4'-biphenyldicarboxylic acid
ActiveCN103483186BAtom economy is highHigh yieldOrganic compound preparationCarboxylic preparation by oxidationChemical synthesisSynthesis methods
The invention relates to the field of chemical synthesis, and in particular to a synthesis method for 4,4'-biphenyldicarboxylic acid. The synthesis method for 4,4'-biphenyldicarboxylic acid is characterized by comprising the following reaction steps of: step 1, reacting 4-diphenic acid, oxalyl chloride and aluminium trichloride in a molar ratio of 1: (1.1-1.8): (1.2-2.0) to obtain 4,4'-dicarboxylbibenzil, wherein the weight ratio of 4-diphenic acid to a solvent is 1: (3 to 10); and step 2, reacting 4,4'-dicarboxylbibenzil, an oxidant and potassium hydroxide in a molar ratio of 1: (1.2-2.0):(1.4-2.2), and then carrying out recrystallization by absolute methanol to obtain 4,4'-biphenyldicarboxylic acid, wherein the weight ratio of 4,4'-dicarboxylbibenzil to an organic solvent is 1: (3-10). Compared with the prior, the synthesis method for 4,4'-biphenyldicarboxylic acid disclosed by the invention has the beneficial effects of being reasonable and practicable in process flow, high in yield which is up to move than 93%, moderate in process reaction conditions, low in requirements on equipment, and suitable for industrialized production.
Owner:SINOSTEEL ANSHAN RES INST OF THERMO ENERGY CO LTD
Process for preparing 4'-trifluoro methyl-2-diphenyl formic acid
InactiveCN100413843CRaw materials are cheap and easy to getLow pricePreparation from nitrilesGrignard reagentDiphenic acid
The present invention discloses process of preparing 4'-trifluoro methyl-2-diphenic acid. The process includes the following steps: 1. the reflux reaction of p-chloro trifluorotoluene and magnesium chip in anhydrous tetrahydrofuran under nitrogen protection to obtain Grignard reagent; 2. mixing o-chloro phenyl nitrile, 1, 2-bis(diphenyl phosphine) ethane nickel chloride and tetrahydrofuran via stirring; dropping the Grignard reagent; filtering and eliminating solvent to recover tetrahydrofuran; adding toluene and water solution of ammonium chloride; and oil-water separating to obtain toluene solution of 4'- trifluoro methyl-2-cyan diphenyl; and 3. heating the toluene solution in alkaline water solution to hydrolyze; oil-water separation to obtain water solution of 4'-trifluoro methyl-2-diphenic sodium and further treatment to obtain 4'-trifluoro methyl-2-diphenic acid. The process has high yield, low cost and other advantages, and is suitable for industrial production.
Owner:ZHEJIANG UNIV
A high-barrier composite film
ActiveCN106432898BImprove flame retardant performanceImprove antibacterial propertiesSynthetic resin layered productsCoatingsBenzodioxolesFluosilicates
The invention discloses a cast film with a high barrier property. PP plastic particles and PET plastic particles are added into a co-extruding film casting machine to prepare a PET / PP film containing a PET layer and a PP layer. The PP plastic particles are prepared through blending modification, and N,N'-(4,4'-methylene diphenyl)bismaleimide, trimethylolpropane trimethacrylate and 4,4'-dihydroxy diphenyl sulfide are added to improve the barrier property of the cast film. The PET plastic particles are modified through a synthetic modification method, and comprise 2-nitroterephthalic acid-4-methyl ester, maleic anhydride, 2-methyl-2,4-pentanediol, 2,3-diobromoethane-1,4-glycol, tetrabromo terephthalic acid, bis(2-ethyl hexyl)terephthalic acid, 4-phthalic anhydride, 2-amino terephthalic acid, 10 parts of 2,2-diemthyl-1,3-propylene glycol, 2,2-difluoro-1,3-benzodioxole, magnesium fluosilicate and antibacterial agent with terephthalic acid and ethylene glycol being base materials. Through complementation of the two materials, the good effect of high barrier, flame retardancy and antibacterium is achieved.
Owner:CANGNAN BAOFENG PRINTING
Method for preparing diphenic acid
InactiveCN100336793CSimple methodDoes not harm the ecological environmentOrganic compound preparationCarboxylic preparation by oxidationAcetic acidPhenanthrene
Owner:COUNCIL OF SCI & IND RES
4-diphenyl formate/leuh nanometer complex and synthetic method
InactiveCN102199423BImprove stabilityImprove propertiesLuminescent compositionsEnergy transferFormate
The invention provides 4-diphenyl formate / LEuH nanometer complex and a synthetic method thereof. The synthetic method comprises the following steps of: a) dissolving EuCl3.6H2O, NaCl and hexamethylenetetramine (HMT) in water, and introducing N2 to perform a hydro-thermal reaction, filtering, washing, and drying to synthesize Eu(OH)2.5Cl0.5.nH2O; and b) adding NaOH into 4-diphenic acid to obtain sodium salt solution of the 4-diphenic acid, dispersing the Eu(OH)2.5Cl0.5.nH2O prepared in the step a) in the sodium salt solution of the 4-diphenic acid to perform the hydro-thermal reaction, and washing and drying, wherein the molar number of the 4-diphenic acid is 3 times that of the Eu(OH)2.5Cl0.5.nH2O. In the synthetic method, 4-diphenyl formate is inserted among LEuH layers to improve the efficiency of energy transfer, the stability of the synthesized 4-diphenyl formate / LEuH nanometer complex is high, and the luminous performance of Eu<3+> is enhanced, so the complex is a lamellate composite material with excellent performance.
Owner:BEIJING NORMAL UNIVERSITY +1
Preparation method of 2,2'-diamido-diphenic acid
InactiveCN108558671AEasy to separateReduce lossesOrganic compound preparationAmino-carboxyl compound preparationPotassium hydroxideDiphenic acid
The invention discloses a preparation method of 2,2'-diamido-diphenic acid. The preparation method comprises the following steps: firstly, mixing [1,1'-biphenyl]-4,4'-dimethyl dicarboxylate with a reaction solution C, stirring and reacting under the condition of ice bath, reacting at room temperature and adding ice blocks; after extracting, washing with saturated salt water, drying with anhydroussodium sulfate, and carrying out decompressing and spin drying to obtain a compound A; secondly, mixing the compound A with methanol for stirring, adding palladium carbon, displacing the air with hydrogen, stirring at room temperature, removing the palladium carbon by filtering and carrying out decompression concentration to obtain a compound B; thirdly, dissolving the compound B in tetrahydrofuran, adding double distilled water and potassium hydroxide, stirring at room temperature, washing with organic phase for two times and combining the water phase with water for cleaning the organic phaseto obtain a mixed solution F; adjusting the pH value of the mixed solution F to 3 by using concentrated hydrochloric acid, filtering and carrying out air drying to obtain the 2,2'-diamido-diphenic acid. The preparation method of the 2,2'-diamido-diphenic acid disclosed by the invention has the advantages of simplicity, high yield and low production cost.
Owner:GUANGDONG MEDICAL UNIV
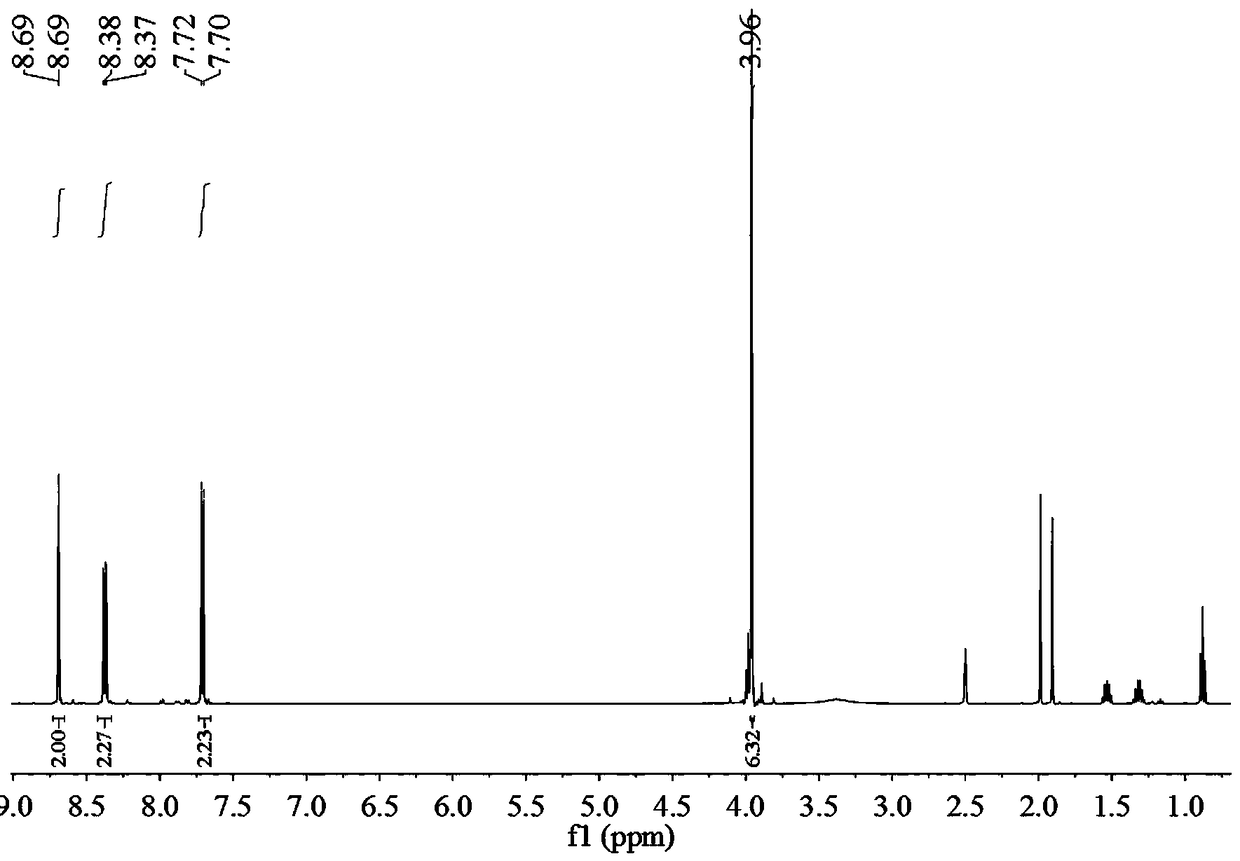
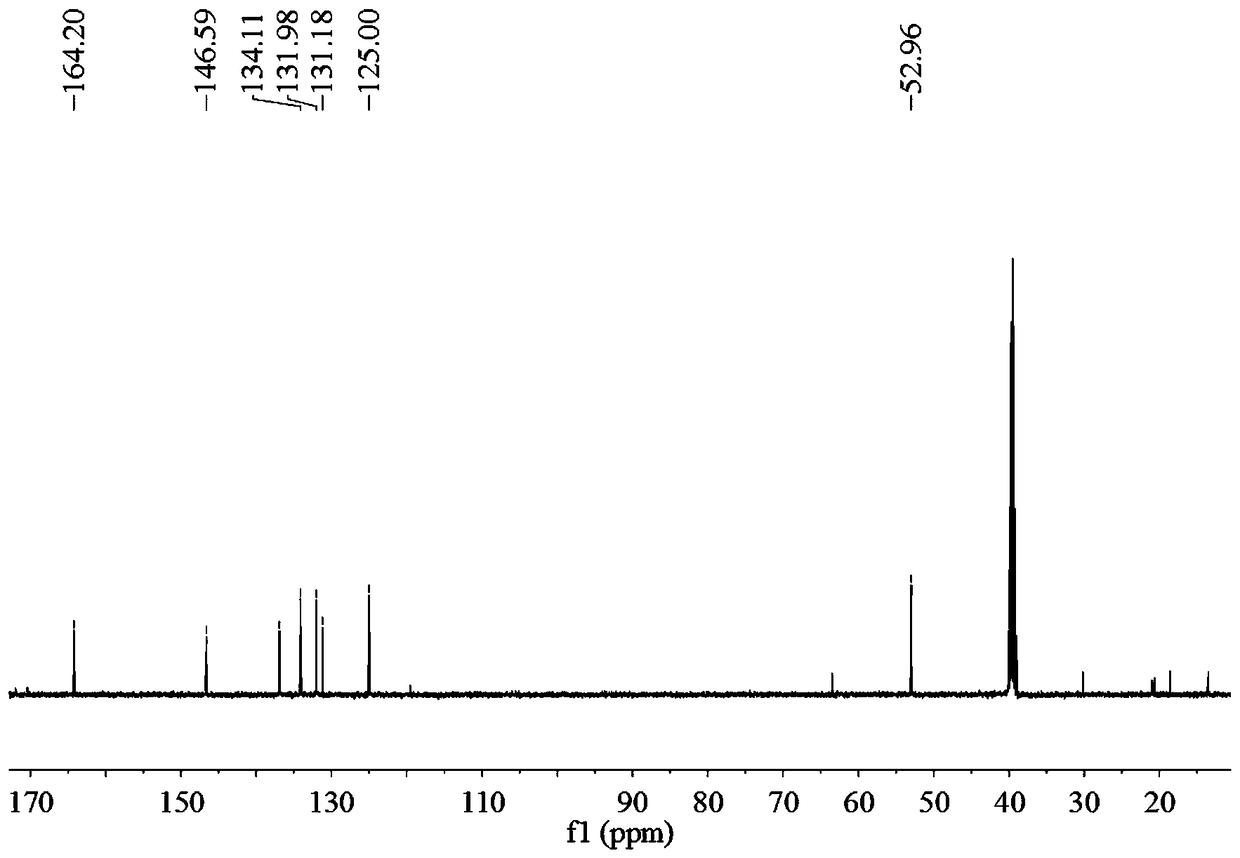
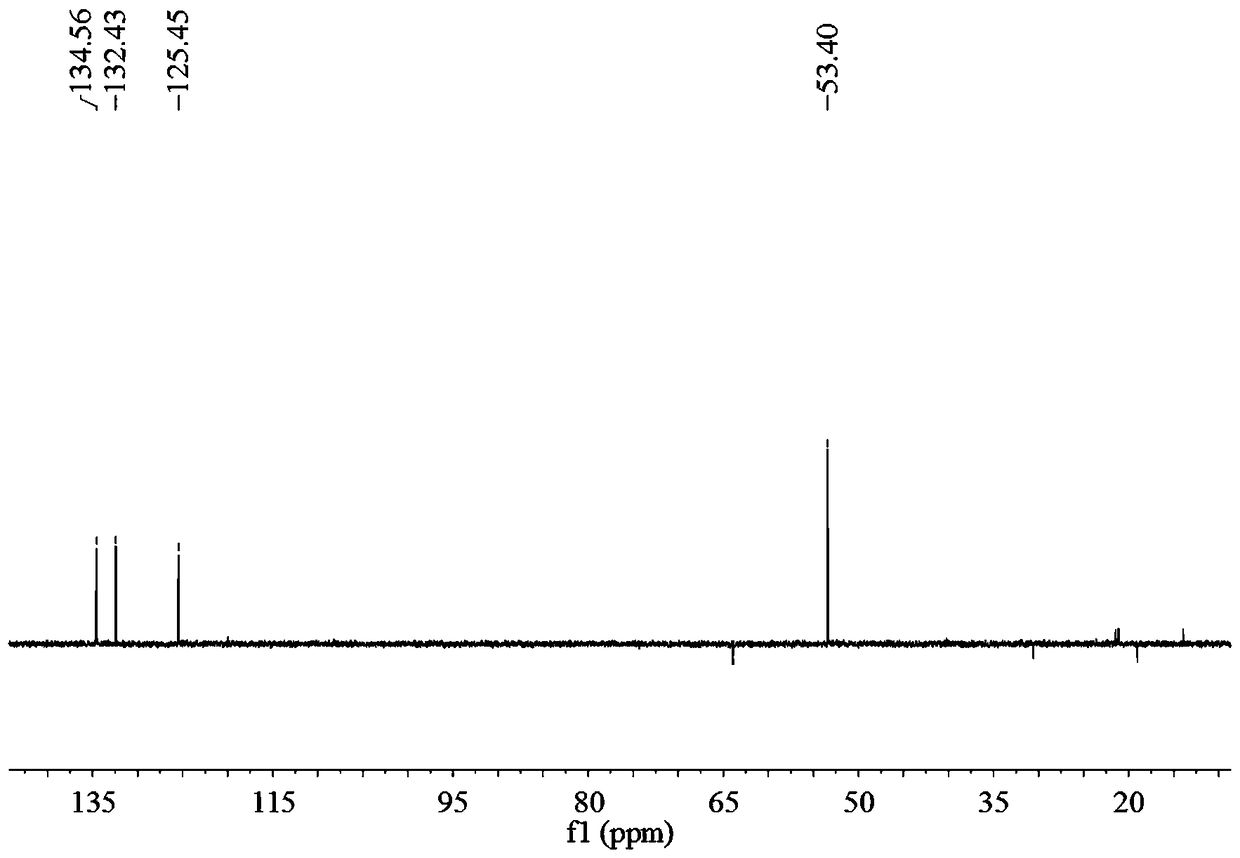
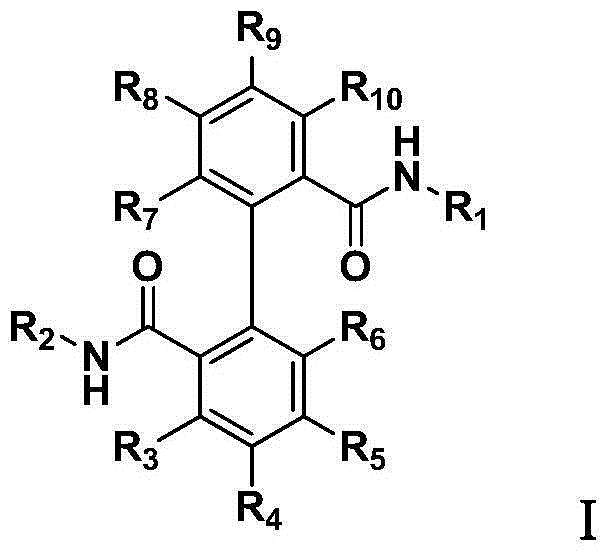
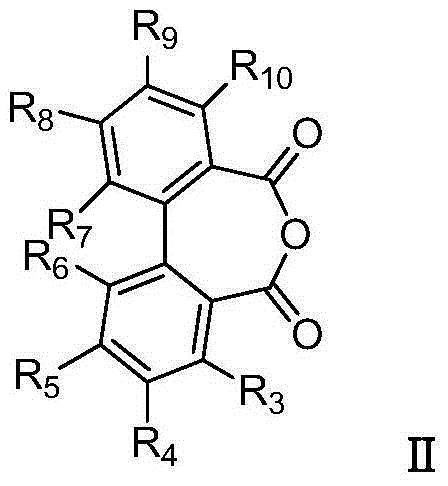
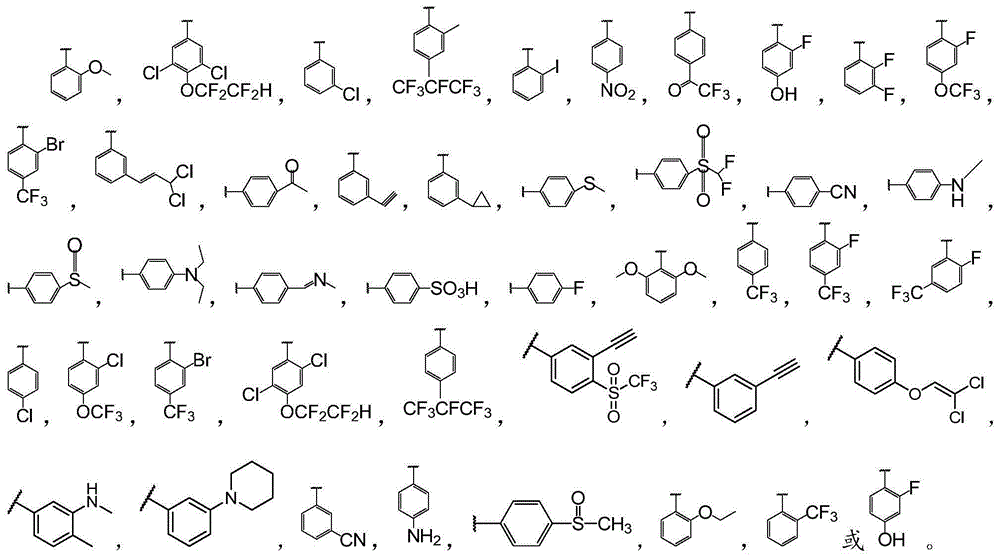
Biphenyl bisamide compound as well as preparation method and application thereof
The invention relates to a biphenyl bisamide compound as well as a preparation method and application thereof. The biphenyl bisamide compound is prepared by reacting 2,2'-diphenic acid anhydride or a derivative thereof with corresponding amines (H2NR1 and H2NR2) respectively. The biphenyl bisamide compound provided by the invention has remarkable insecticidal activity and a selective insecticidal spectrum, is simple in preparation process, and is expected to be developed into a novel pesticide which is low in toxicity, efficient and environment-friendly.
Owner:EAST CHINA UNIV OF SCI & TECH
Europium Fluorescent Probe and Test Paper Based on Diphenyldicarboxylic Acid and Its Application in Detecting p-Phenylenediamine
ActiveCN107057685BBright redDistinct characteristic emission peaksGroup 3/13 organic compounds without C-metal linkagesFluorescence/phosphorescenceDiphenic acidCrystal cell
The invention discloses an Europium fluorescent probe and test paper based on diphenic acid and application thereof in the detection of p-phenylenediamine, the structural unit of the fluorescent probe is [PMI]2[Eu2(BPDC)3C12], wherein the PMI represents 1-propyl-3-methylimidazole monovalent cation, BPDC represents diphenic acid radical that loses protons on two carboxyl groups; the fluorescent probe belongs to a monoclinic crystal system and the space group is P21 / c. the crystal cell parameters are as follows: alpha is equal to gamma, which is equal to 90 degree, belta is equal to 96.6830(10) degree; and the crystal cell volume of z is equal to 4. The fluorescent probe of the invention has excellent red fluorescence properties and shows bright red under the irradiation of an ultraviolet lamp, the p-phenylenediamine in the aqueous phase has a significant quenching effect on the red fluorescence of the fluorescent probe and test paper, so the fluorescent probe and test paper can be used for detecting p-phenylenediamine with high sensitivity, high selectivity and anti-interference, the fluorescent probe and test paper are disposable (similar to normal pH test paper).
Owner:SHAANXI NORMAL UNIV
A kind of polyester resin for anti-yellowing haa system powder coating, preparation method and application
ActiveCN109054008BImprove high temperature resistanceImprove anti-yellowing effectPowdery paintsPolyester coatingsPolymer scienceHexamethylenediamine
The invention belongs to the technical field of resin production, and particularly relates to a polyester resin for an anti-yellowing HAA (N,N,N',N'-tetra(Beta-hydroxyethyl)adipamide) system and further relates to a preparation method and application of the polyester resin. The anti-yellowing HAA system provided by the invention is polymerized by using pyromellitic dianhydride, 1,6-hexanediamine,isophthalic acid, phloroglucinol formic acid, ethanetricarboxylic acid monoester, 2,5-dihydroxy hexane, neopentyl glycol and 2,2'-diphenic acid. Because a polyimide chain is carried in the molecular chain of the polyester resin obtained by the materials and a specific process, the high temperature-resistant and anti-yellowing properties are excellent; moreover, because the materials containing phenolic hydroxyl groups, such as phloroglucinol formic acid and ethanetricarboxylic acid monoester, are used for participating in reaction and the phenolic functional group with high anti-yellowing capability is contained in the polyester chain, the polyester product has excellent high temperature-resistant and anti-yellowing properties, and is ultimately used in the powder paint of the HAA system,so that a coated panel with excellent anti-yellowing property can be obtained.
Owner:HUANGSHAN HUIZHOU KANGJIA CHEM CO LTD
Synthetic method for 4,4',6,6'-tetranitro-2,2'-diphenic acid
The invention relates to a method for preparing 4,4',6,6'-tetranitro-2,2'-diphenic acid. According to the method, 2,2'-diphenic acid is used as a raw material directly; and 4,4',6,6'-tetranitro-2,2'-diphenic acid is obtained by a mixed acid nitration method. The method is simple in process and high in yield, and is suitable for production.
Owner:INNER MONGOLIA UNIV OF TECH
Asymmetric diphenic acid isomeride separation method
InactiveCN105037134AMild reaction conditionsSimple and fast operationOrganic compound preparationCarboxylic compound preparationDiphenic acidAqueous solution
The invention discloses an asymmetric diphenic acid isomeride separation method, which includes the steps of mixing mixed acid and inorganic alkali in a water solution, stirring the solution to clarify the solution to obtain metal salt, performing heating reflux to distill partial water out to turbid the solution, cooling the solution to precipitate-out solid, re-dissolving the solid in the water solution, and performing acidification to obtain the product. The method is mild in reaction conditions, is simple in operation and is good in separation effect.
Owner:启东东岳药业有限公司 +1
A double-layer co-extruded cast film
ActiveCN106046539BImprove flame retardant performanceImprove antibacterial propertiesSynthetic resin layered productsCoatingsComposite filmPolymer science
The invention discloses a double-layer coextrusion casting film which comprises a PET layer and a PP layer. PP plastic particles comprise PP, trihydroxy polyoxypropylene ether, N,N'-(4,4'-methylene diphenyl)bismaleimide, trimethylolpropane trimethacrylate, 4,4'-dyhydroxyl diphenyl sulfide, montmorillonite, paraffin, antioxidant 1010, nano TiO2, polyether siloxane copolymer, methyl hydroxyethyl cellulose and VTPS silane coupling agent. PET plastic particles comprise terephthalic acid, ethylene glycol, maleic anhydride, 2-nitroterephthalic acid-4-methyl ester, 2,2-dimethyl-1,3-propylene glycol, 2,3-dibromo-2-butene-1,4-glycol, 1,4-benzene dimethanol, 2-aminoterephthalic acid, tetrabromo terephthalic acid, bis(2-ethyl hexyl)terephthalic acid, 4-bromo phthalic anhydride, 2,2-difluoro-1,3-benzodioxole, magnesium fluosilicate and antibacterial agent. By the combining of the PP layer and the PET layer, the whole composite film is high in barrier property, antibacterial and flame-retardant.
Owner:江苏求实塑业有限公司
Method for separating diphenic acid from tower bottom waste for preparing benzoic acid
InactiveCN102336659AExpand the scope of useEasy to separate and purifyCarboxylic compound separation/purificationBenzoic acidOrganic solvent
The invention provides a method for separating diphenic acid from tower bottom waste for preparing benzoic acid, and in particular relates to a method for extracting mixed diphenic acid from tower bottom waste for preparing benzoic acid by direct liquid phase oxidation of toluene and separating and purifying 3-phenyl benzoic acid and 4-phenyl benzoic acid. Any high-toxicity organic solvent and any catalyst are not used in the reaction process; the purification process is simple in operation; and the toluene serving as an organic solvent in the reaction process and the purification process canbe recycled in a rectifying mode, and ethanol serving as an organic solvent can be recycled by rectification after simple treatment. The method further expands the utilization range of the tower bottom waste for preparing the benzoic acid by direct liquid phase oxidation of the toluene, and has the advantages of simplicity and convenience in operation, easiness in separation and purification of the product, low environmental pollution, low cost, high economic value and the like.
Owner:SHANXI UNIV
4-diphenyl formate/leuh nanometer complex and synthetic method
InactiveCN102199423AImprove stabilityImprove propertiesLuminescent compositionsEnergy transferHexamethylenetetramine
Owner:BEIJING NORMAL UNIVERSITY +1
Method for separating diphenic acid from tower bottom waste for preparing benzoic acid
InactiveCN102336659BEasy to separate and purifyReduce pollutionCarboxylic compound separation/purificationBenzoic acidOrganic solvent
The invention provides a method for separating diphenic acid from tower bottom waste for preparing benzoic acid, and in particular relates to a method for extracting mixed diphenic acid from tower bottom waste for preparing benzoic acid by direct liquid phase oxidation of toluene and separating and purifying 3-phenyl benzoic acid and 4-phenyl benzoic acid. Any high-toxicity organic solvent and any catalyst are not used in the reaction process; the purification process is simple in operation; and the toluene serving as an organic solvent in the reaction process and the purification process can be recycled in a rectifying mode, and ethanol serving as an organic solvent can be recycled by rectification after simple treatment. The method further expands the utilization range of the tower bottom waste for preparing the benzoic acid by direct liquid phase oxidation of the toluene, and has the advantages of simplicity and convenience in operation, easiness in separation and purification of the product, low environmental pollution, low cost, high economic value and the like.
Owner:SHANXI UNIV
4-nor-amyl diphenic acid-3-fluor-4-cyan phenol ester production process
ActiveCN100374413CThe reaction conditions are reasonable and feasibleReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationSteam pressureDistillation
This invention relates to a method for producing 4-amyl-biphenylcarboxylic acid-3-fluoro-4-cyanophenol ester, comprising: amyl-biphenylcarboxylic acid, thionyl chloride, 3-fluoro-4-cyanophenol, toluene, sodium-hydroxide, and anhydrous ethyl alcohol as raw material, with mole ration as: amyl-biphenylcarboxylic acid 1.05, thionyl chloride 2.65, 3-fluoro-4-cyanophenol 1, toluene 3, sodium-hydroxide 0.1, and anhydrous ethyl alcohol 4.0. The process comprises two steps: (1) use amyl-biphenylcarboxylic acid and thionyl chloride as raw material for acylation reaction and synthesize amyl-bibenzoyl chloride by normal pressure and decreasing pressure distillation; (2) dropwise add amyl-bibenzoyl chloride and 3-fluoro-4-cyanophenol with toluene as solvent for esterification, and produce the target product 4-amyl-biphenylcarboxylic acid-3-fluoro-4-cyanophenol ester by water washing, alkaline washing, crystallization and recrystallization, wherein the conditions: acylation reaction temperature 160Deg C, reflux time 6h, normal distillation temperature 172-176Deg C, pressure decreasing temperature 118-121Deg C, vacuum 50mmHg, the discharging condition is to collect the 3Deg C range product after temperature and pressure stabilized, the temperature of adding 3-fluoro-4-cyanophenol with agitation for acylation reaction is 100Deg C, keep the temperature between 90 and 100Deg C when dropwise adding amyl-bibenzoyl chloride for 3h, reflux with agitation for 6h, heating steam pressure no more than 0.2MPa, autoclave inside temperature kept at 120+2Deg C, crystallization temperature -15Deg C and kept at -15+-2Deg C for 1h for agitation, recrystallization temperature -15Deg Cand kept at -15+-2Deg C for 1h for agitation.
Owner:江苏和纯化学工业有限公司
Biphenyl bisamide compounds and their preparation and use
InactiveCN103772228BNovel structureBiocideCarboxylic acid nitrile preparationDiphenic acidPerylene derivatives
The invention relates to a biphenyl bisamide compound as well as a preparation method and application thereof. The biphenyl bisamide compound is prepared by reacting 2,2'-diphenic acid anhydride or a derivative thereof with corresponding amines (H2NR1 and H2NR2) respectively. The biphenyl bisamide compound provided by the invention has remarkable insecticidal activity and a selective insecticidal spectrum, is simple in preparation process, and is expected to be developed into a novel pesticide which is low in toxicity, efficient and environment-friendly.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com