Preparation method of 2,2'-diamido-diphenic acid
A technology of biphenyl dicarboxylic acid and diamino acid, which is applied in the field of preparation of 2,2'-diamino-biphenyl dicarboxylic acid, can solve the problems of high product loss, complicated preparation method and high production cost, and achieves the advantages of simple preparation method, Simple preparation method and less product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
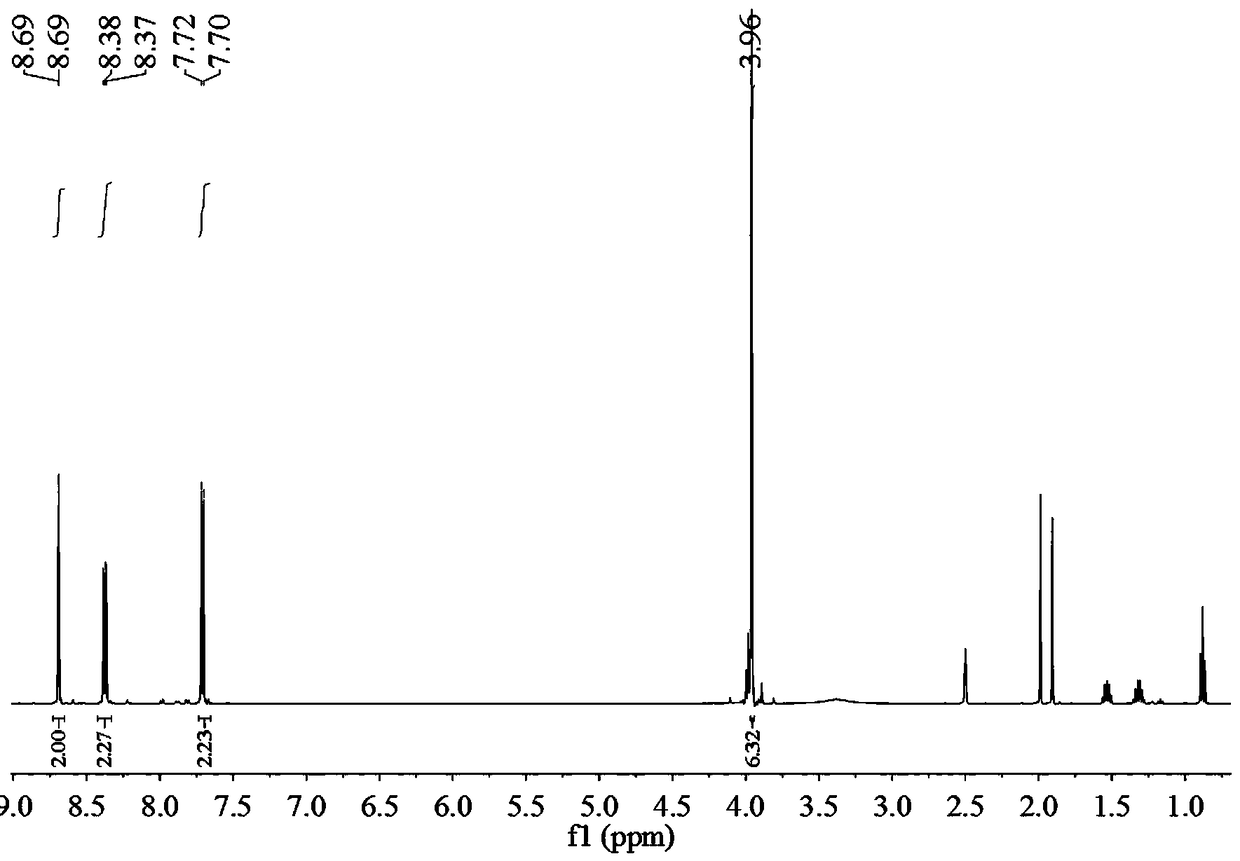
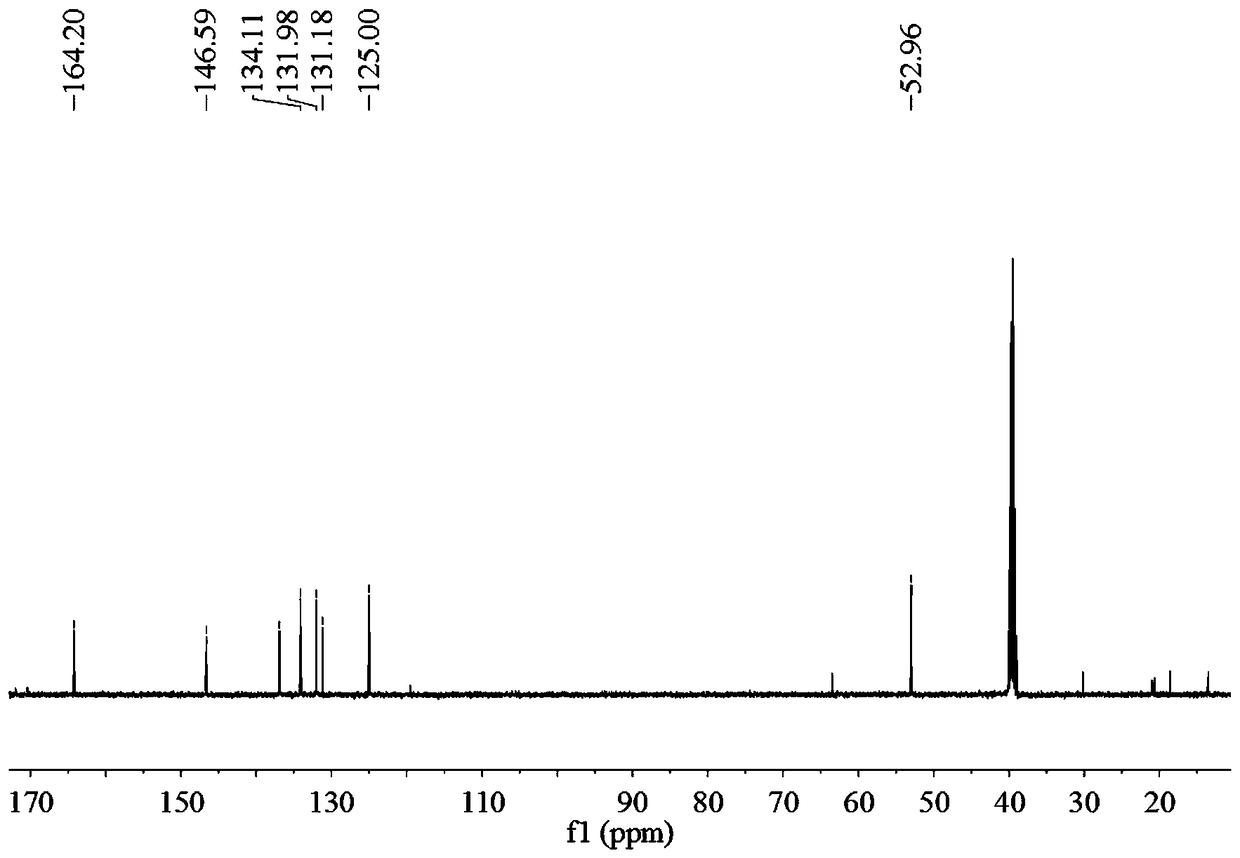
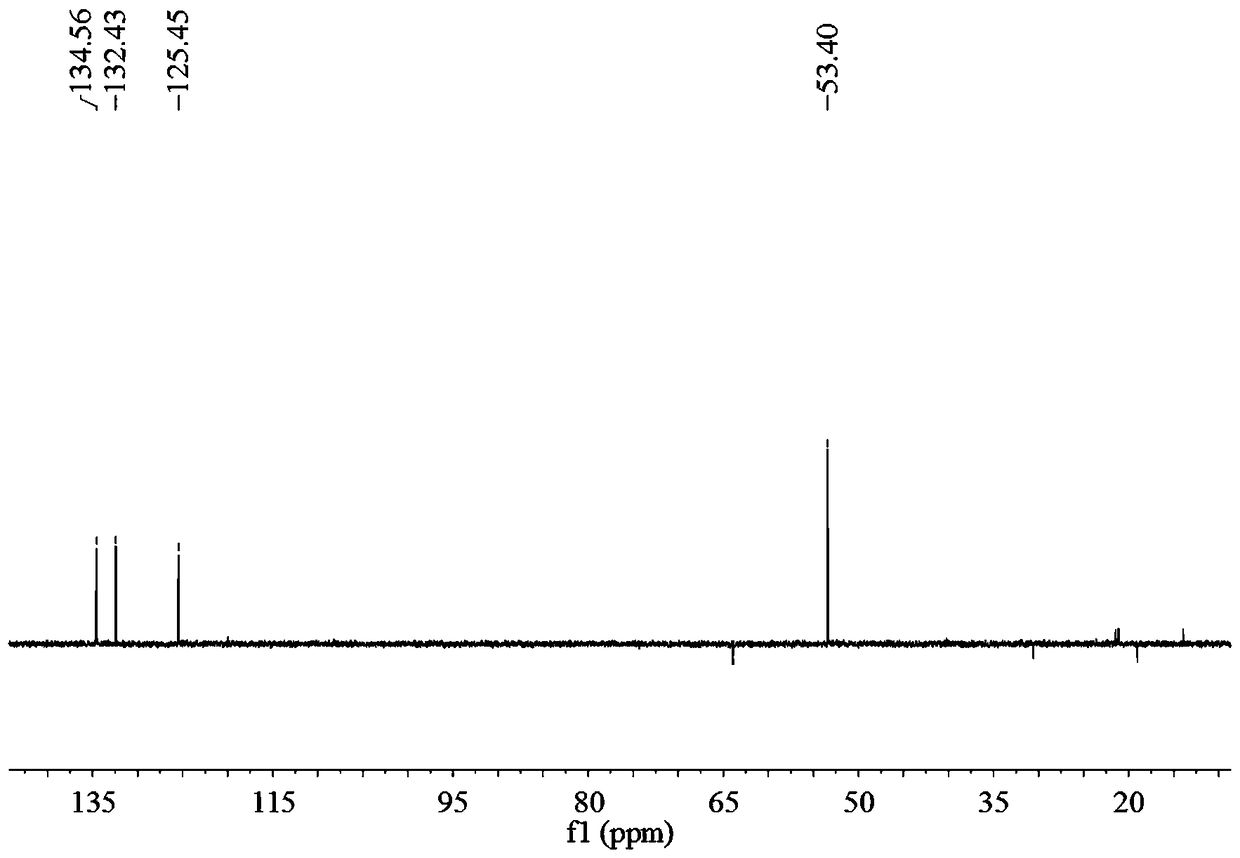
[0036] A method for preparing 2,2'-diamino-diphthalic acid, including the following steps:
[0037] 1) Preparation of compound A: Put 1.35g [1,1'-biphenyl]-4,4'-dimethyl dicarboxylate and reaction liquid C in the reaction flask, and stir for 25min under ice bath conditions. [1,1'-Biphenyl]-4,4'-Dimethyl dicarboxylate is dissolved in reaction solution C, and the color of reaction solution C changes to yellow, forming mixed solution D, with a reaction flask containing mixed solution D inside Continue the reaction for 1 h under ice bath conditions, then at room temperature for 12 h, then add ice cubes to the mixed solution D, extract with dichloromethane and ethyl acetate, combine the filtrate, wash with saturated brine, and then Dry with anhydrous sodium sulfate, and finally spin-dry under reduced pressure to obtain 1.728 g of yellow solid substance, namely compound A, with a yield of 96%;
[0038] Wherein, the preparation method of the reaction solution C is as follows: under ice b...
Embodiment 2
[0042] A method for preparing 2,2'-diamino-diphthalic acid, including the following steps:
[0043] 1) Preparation of compound A: Put 1.35g [1,1'-biphenyl]-4,4'-dimethyl dicarboxylate and reaction liquid C in the reaction flask, and stir for 35min under ice bath conditions. [1,1'-Biphenyl]-4,4'-Dimethyl dicarboxylate is dissolved in reaction solution C, and the color of reaction solution C changes to yellow, forming mixed solution D, with a reaction flask containing mixed solution D inside Continue the reaction for 3h under ice bath conditions, and then at room temperature for 8h, then add ice cubes to the mixed solution D, extract with dichloromethane and ethyl acetate, combine the filtrate, wash with saturated brine, and then Dry with anhydrous sodium sulfate, and finally spin-dry under reduced pressure to obtain 1.769 g of yellow solid substance, namely compound A, with a yield of 98.3%;
[0044] Wherein, the preparation method of the reaction liquid C is as follows: under ice ...
Embodiment 3
[0048] A method for preparing 2,2'-diamino-diphthalic acid, including the following steps:
[0049] 1) Preparation of compound A: Put 1.35g [1,1'-biphenyl]-4,4'-dimethyl dicarboxylate and reaction solution C in the reaction flask, and stir for 30min under ice bath conditions. [1,1'-Biphenyl]-4,4'-Dimethyl dicarboxylate is dissolved in reaction solution C, and the color of reaction solution C changes to yellow, forming mixed solution D, with a reaction flask containing mixed solution D inside Continue the reaction for 2h under ice bath conditions, then at room temperature for 10h, then add ice cubes to the mixed solution D, extract with dichloromethane and ethyl acetate, combine the filtrate, wash with saturated brine, and then Dry with anhydrous sodium sulfate, and finally spin-dry under reduced pressure to obtain 1.763 g of yellow solid substance, namely compound A, with a yield of 97.9%;
[0050] Wherein, the preparation method of the reaction solution C is as follows: under ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com