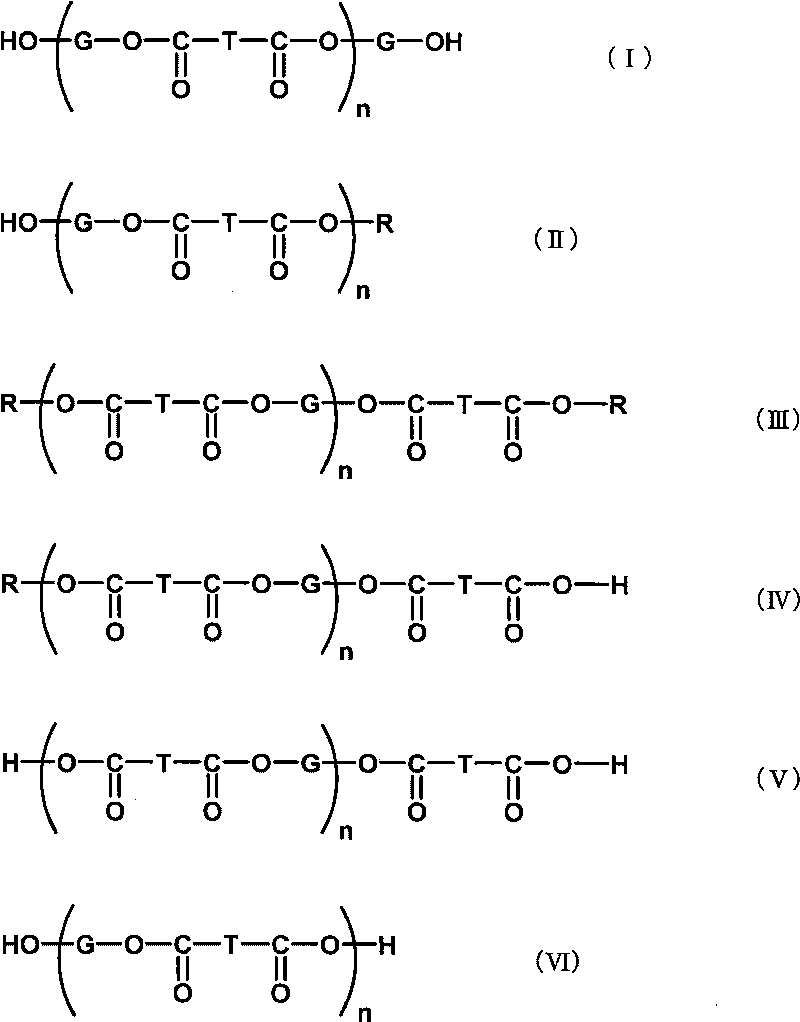Additive for cellulose ester resin, cellulose ester resin composition using same, and film
A technology of cellulose ester resin and additives, applied in the direction of instruments, optics, optical components, etc., to achieve the effect of excellent optical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
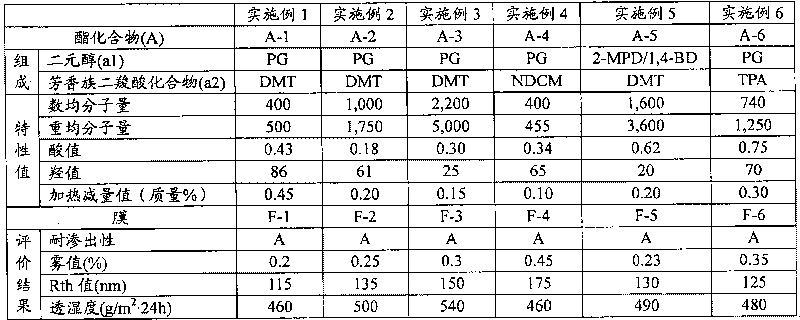
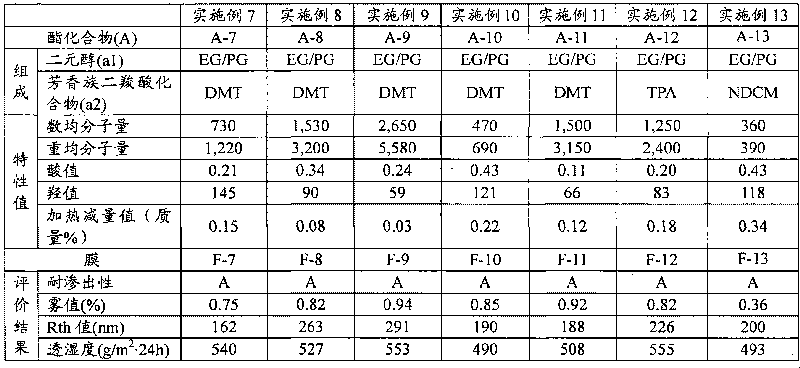
[0109] The manufacture of embodiment 1 ester compound (A-1)
[0110] Add 392 g of 1,2-propanediol (hereinafter referred to as "PG") and dimethyl terephthalate (hereinafter referred to as "DMT") to a four-necked flask with an internal volume of 3 liters equipped with a thermometer, a stirrer and a reflux cooler. ”)800g and heat up. When the temperature in the flask reached 130°C, tetraisopropyl titanate as an esterification catalyst was added at 60 ppm relative to the total amount of PG and DMT, and the generated methanol was distilled off at 185°C while stirring under a nitrogen stream, and the reaction 15 hours. After the reaction, when the temperature in the flask reached 190° C., the pressure was reduced at about 4000 Pa for 2 hours to obtain an ester compound (A-1) (acid value: 0.43, hydroxyl Value: 86).
Embodiment 2
[0111] The manufacture of embodiment 2 ester compound (A-2)
[0112] 940 g of PG and 1,600 g of DMT were put into a four-necked flask with an internal volume of 3 liters equipped with a thermometer, a stirrer, and a reflux cooler, and the temperature was raised. When the temperature in the flask reached 130°C, tetraisopropyl titanate as an esterification catalyst was added at 60 ppm relative to the total amount of PG and DMT, and the generated methanol was distilled off at 185°C while stirring under a nitrogen stream, and the reaction 10 hours. After the reaction, when the temperature in the flask reached 190°C, the pressure was reduced at about 4,000 Pa for 2 hours, and further at about 133 Pa for 1 hour to obtain an ester compound (A- 2) (acid value: 0.18, hydroxyl value: 61).
Embodiment 3
[0113] The manufacture of embodiment 3 ester compound (A-3)
[0114] 1,500 g of the ester compound (A-2) obtained in Example 2 was charged into a four-necked flask with an internal volume of 2 liters, and the temperature was raised. When the temperature in the flask reached 190°C, the pressure was reduced at about 133 Pa for 1.5 hours to obtain an ester compound (A-3) with a number average molecular weight of 2,200 and a weight average molecular weight of 5,000 (acid value: 0.30, hydroxyl value: 25) .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Moisture permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com