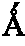Patents
Literature
70 results about "Size selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Membranes with Embedded Nanotubes For Selective Permeability
ActiveUS20100025330A1Good membrane permeabilityHigh selectivityCeramic shaping apparatusReverse osmosisFiltrationNanometre
Membranes for filtration by size exclusion are formed from open-ended nanotubes embedded in a polymeric matrix. The matrix forms a layer whose thickness is substantially less than the average length of the nanotubes, allowing the nanotubes to be randomly oriented throughout the matrix while providing channels extending through the layer for the selective passage of molecular species or particles based on size.
Owner:NAGARE MEMBRANES
Process for preparation of silver nanoparticles, and the compositions of silver ink containing the same
ActiveUS20100189901A1Small sizeGood physical propertiesMaterial nanotechnologyNanostructure manufactureSilver inkAmmonium carbamate
The present invention relates to a process for preparation of silver complex compound and the compositions of silver ink containing the same. The present invention includes a) preparing silver complex compound with special structure by reacting silver compound with at least one or two mixtures selected from ammonium carbamate compound, ammonium carbonate compound or ammonium bicarbonate compound and b) preparing silver nano particles by reacting the silver complex compound with reducer or reducing or pyrolyzing the silver complex compound by applying heat thereto. The preparing method according to the present invention can prepare the silver nano practical with various shapes through a simple preparation process, improve the selectivity of the size of the silver complex compound, fire the silver complex compound even when it is fired at a low temperature of 150° C. or less during a short time, provide the ink compositions capable of forming the coating or the fine pattern showing the high conductivity while facilitating the thickness control of the coating, and provide the ink compositions capable of being applied to the reflective film material, the electromagnetic wave shield, and the antimicrobial agent, etc.
Owner:INKTEC CO LTD
Membranes with embedded nanotubes for selective permeability
InactiveUS20090321355A1Improve permeabilityHigh selectivitySemi-permeable membranesMembranesFiltrationNanometre
Membranes for filtration by size exclusion are formed from open-ended nanotubes embedded in a polymeric matrix. The matrix forms a layer whose thickness is substantially less than the average length of the nanotubes, allowing the nanotubes to be randomly oriented throughout the matrix while providing channels extending through the layer for the selective passage of molecular species or particles based on size.
Owner:NANOASIS TECH INC
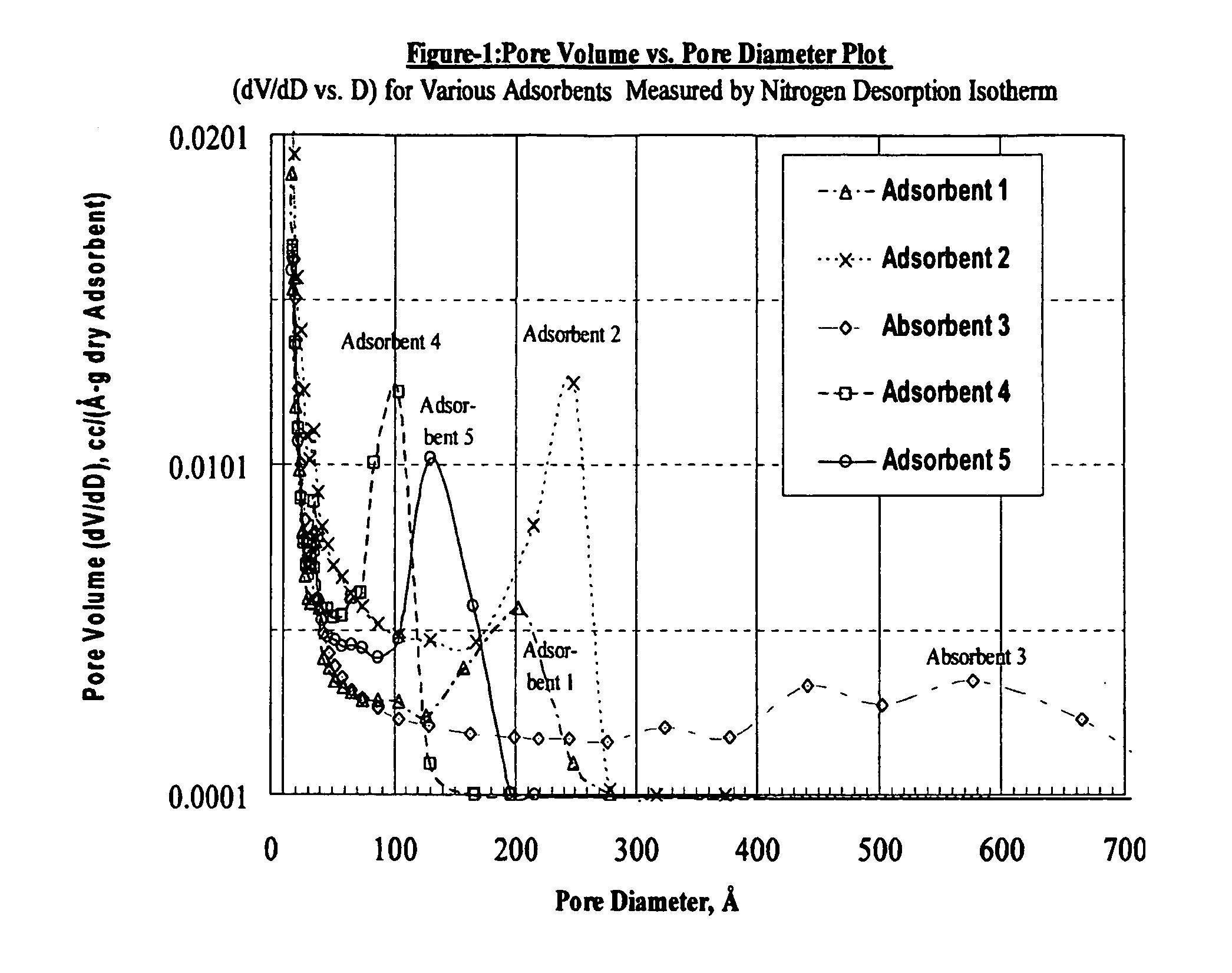
Size-selective hemoperfusion polymeric adsorbents
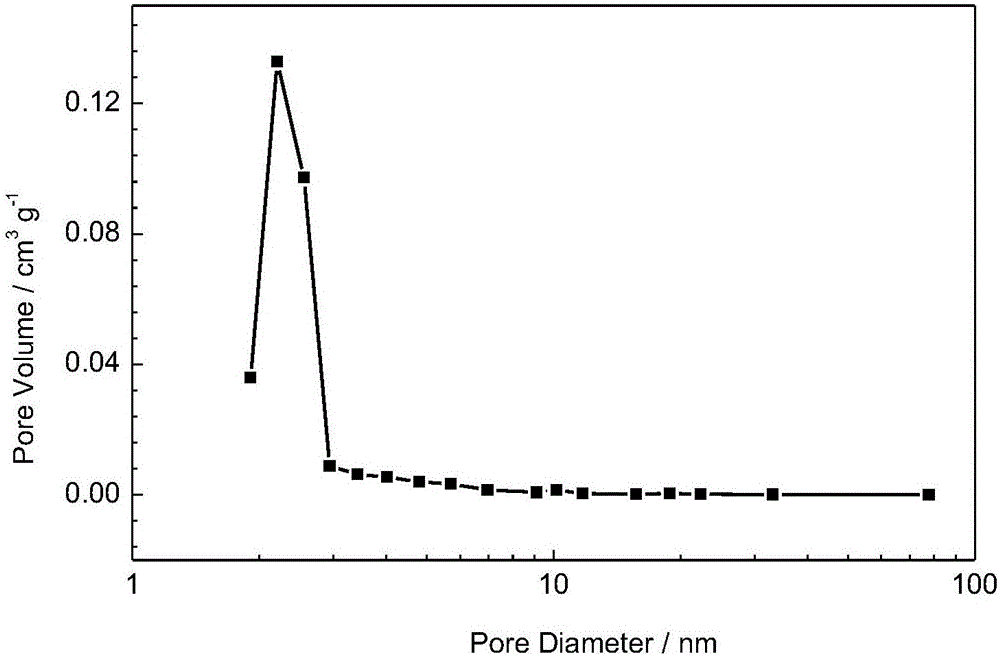
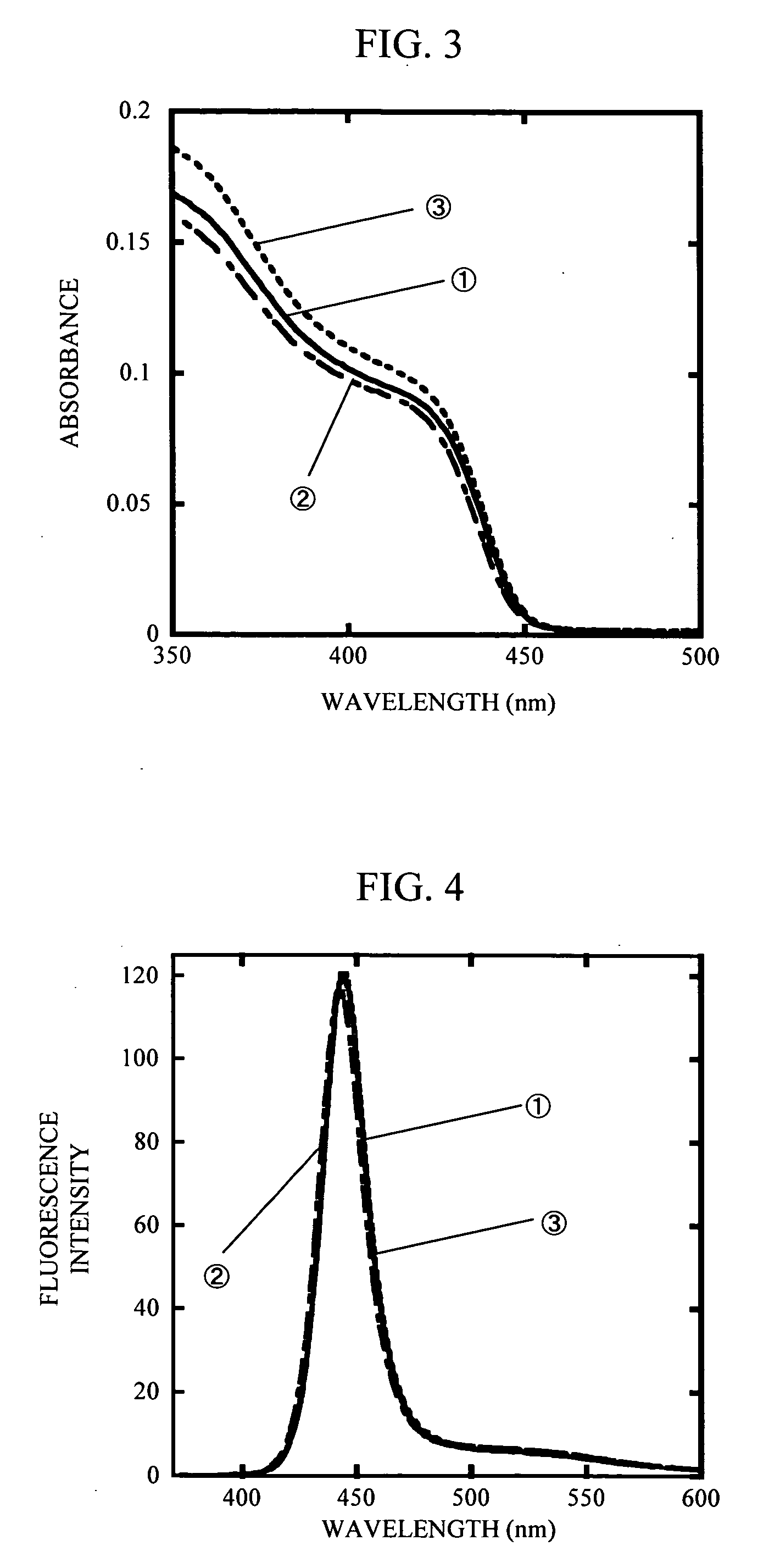
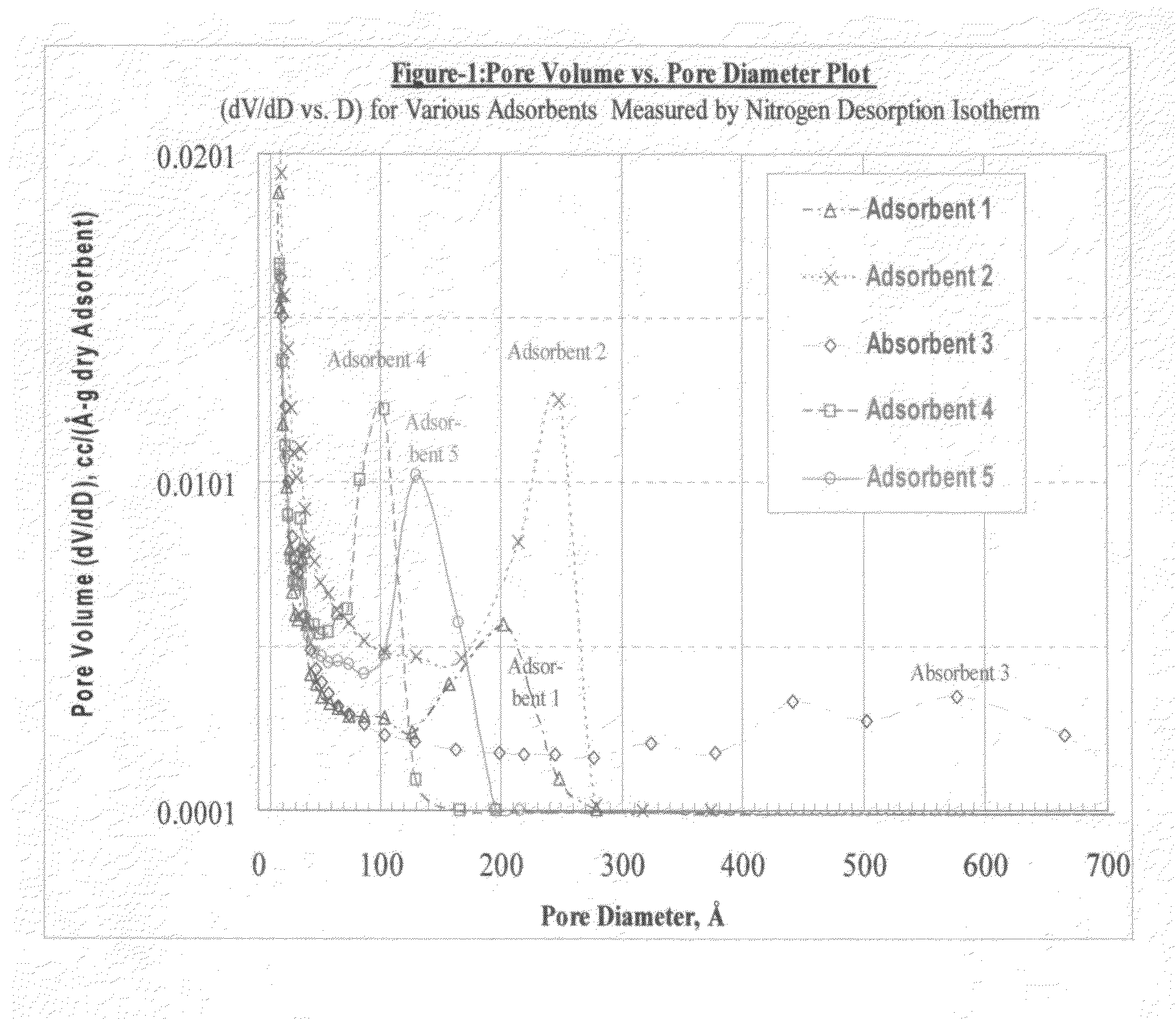
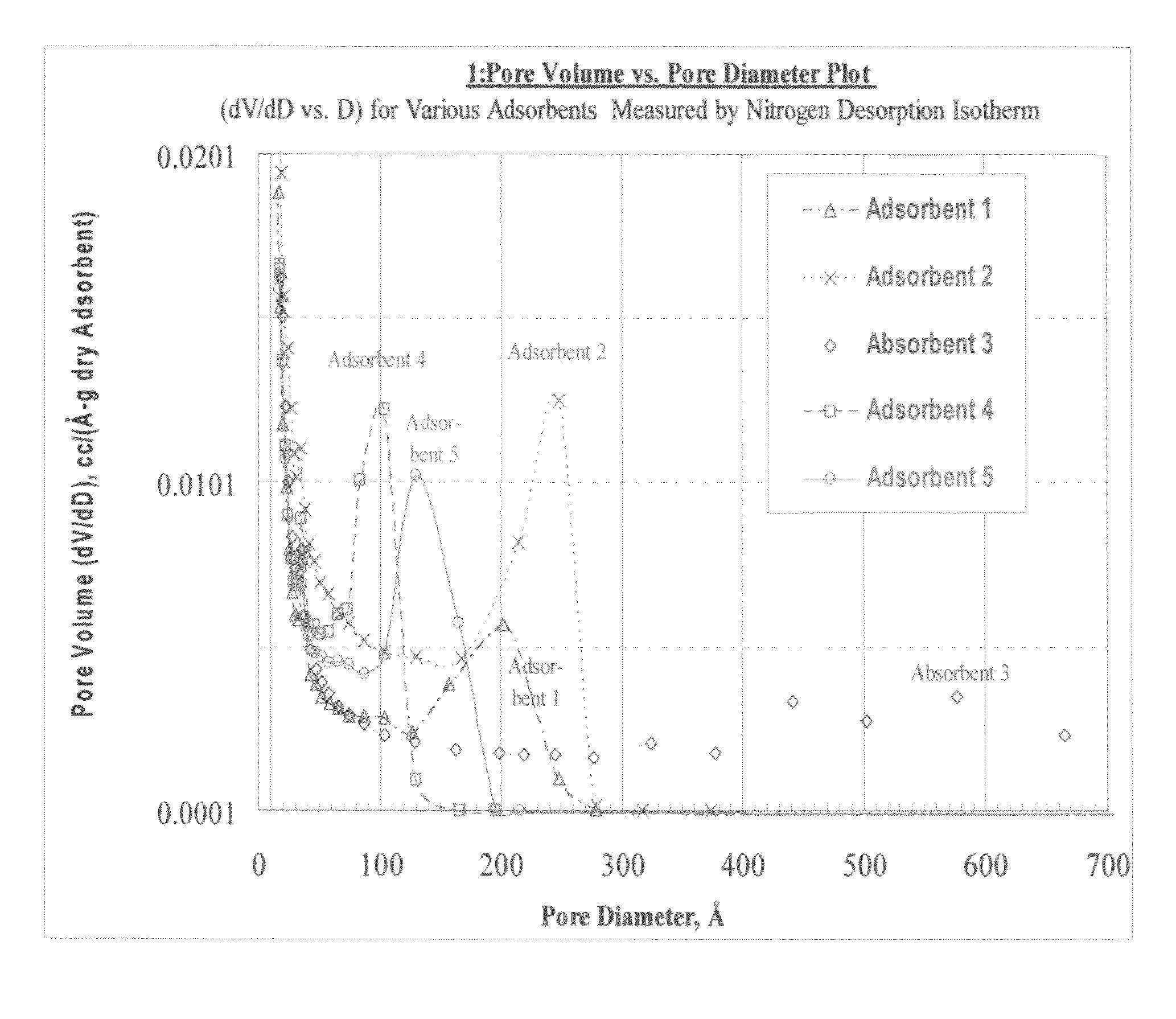
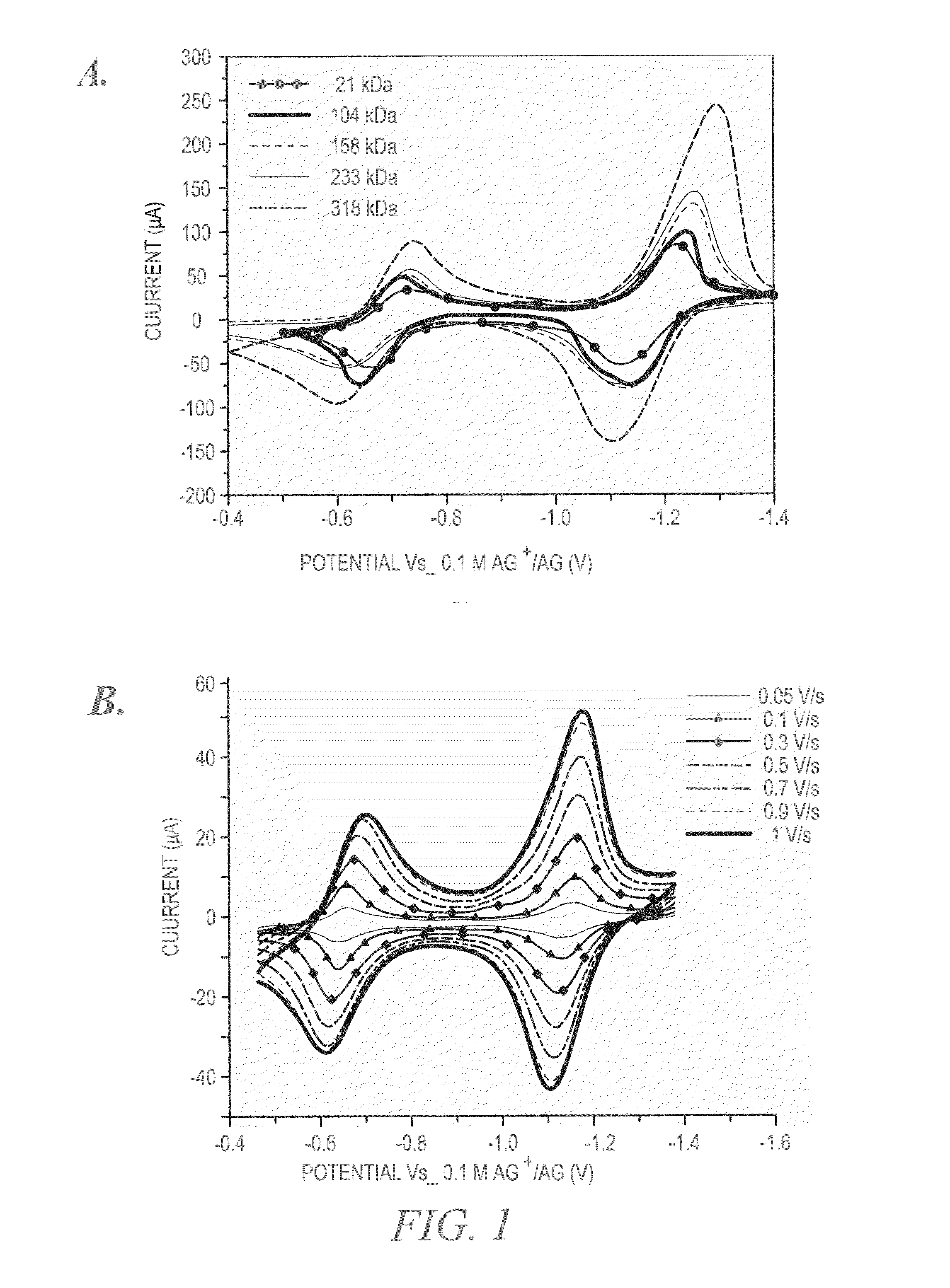
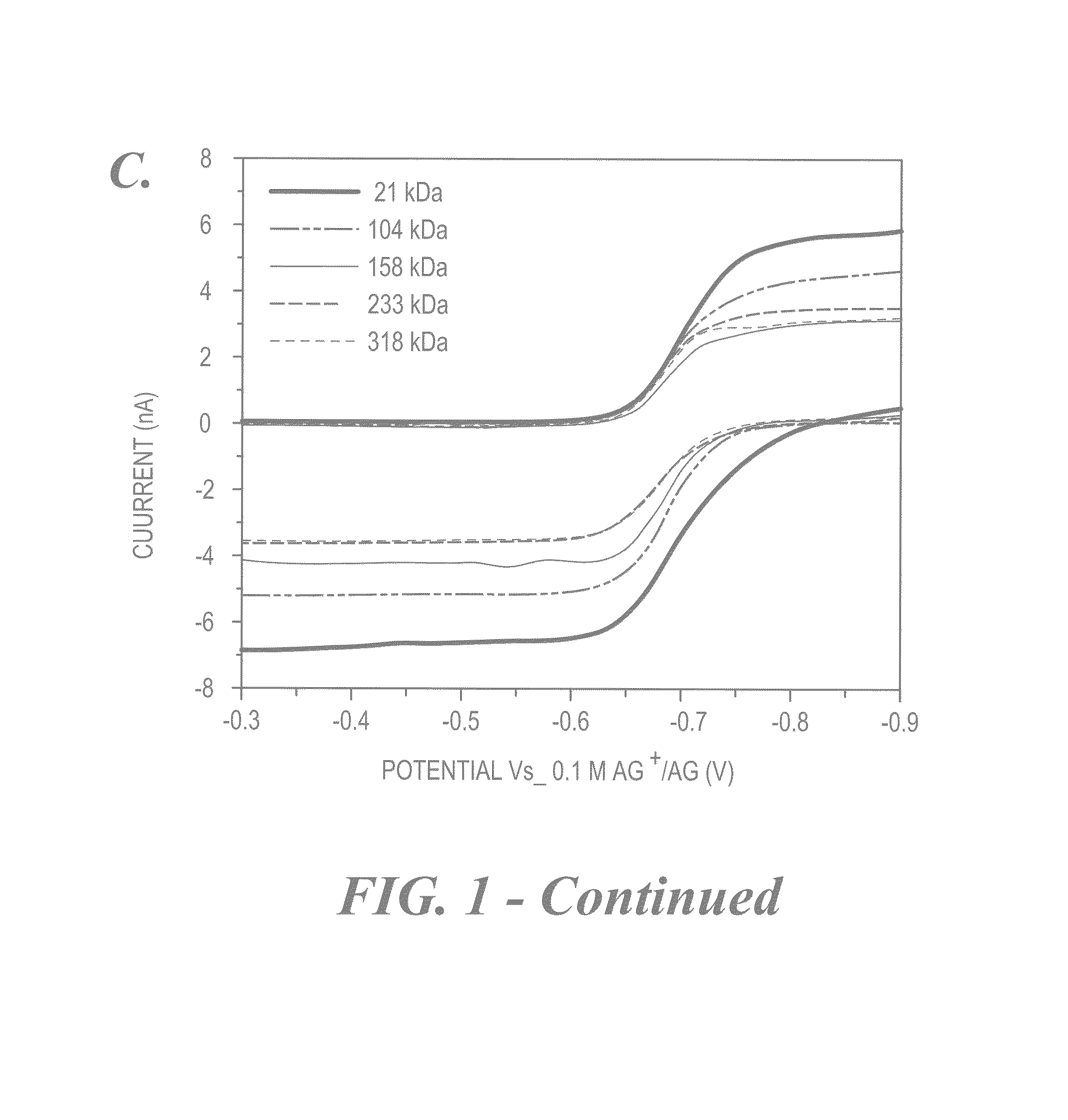
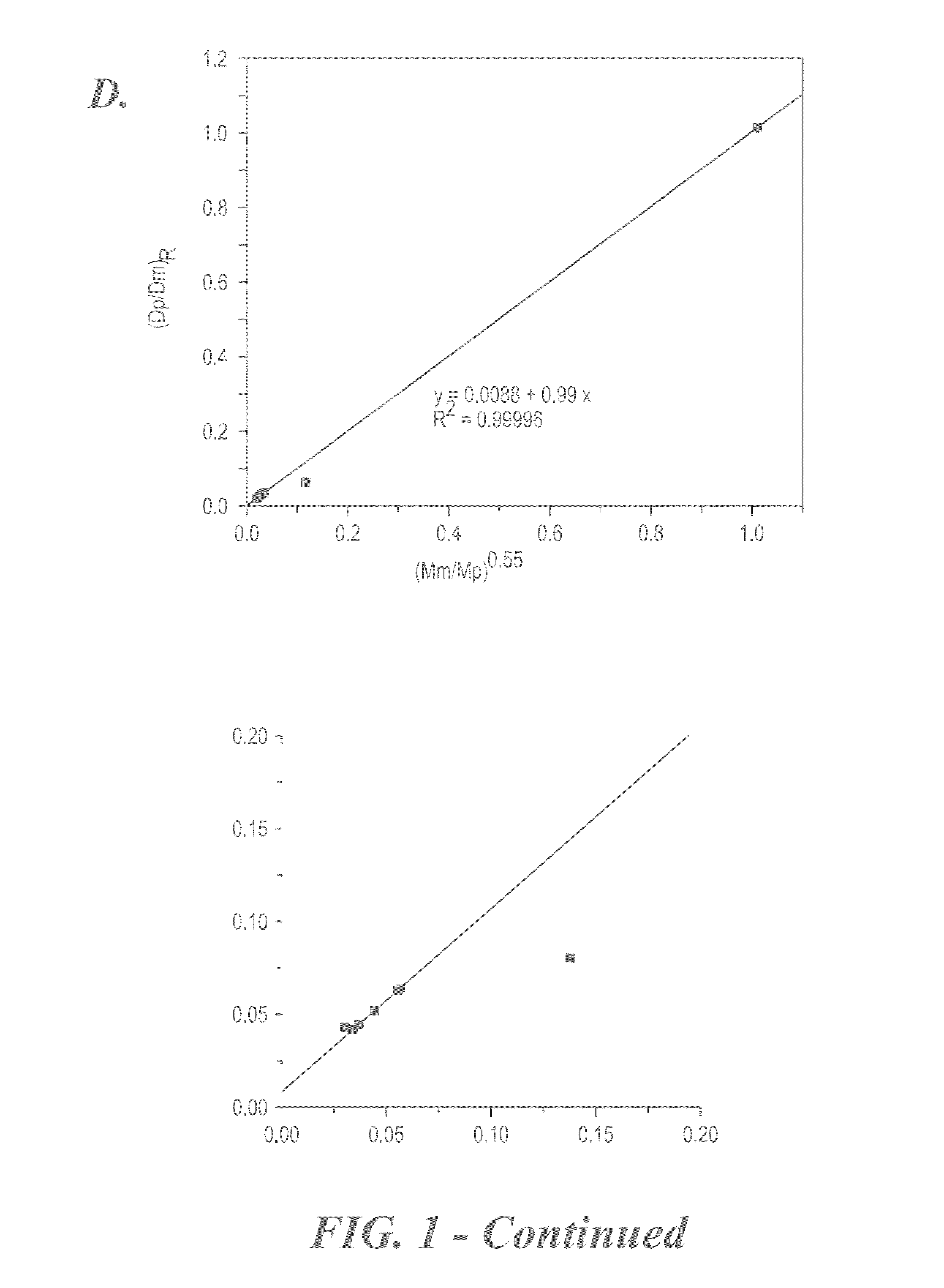
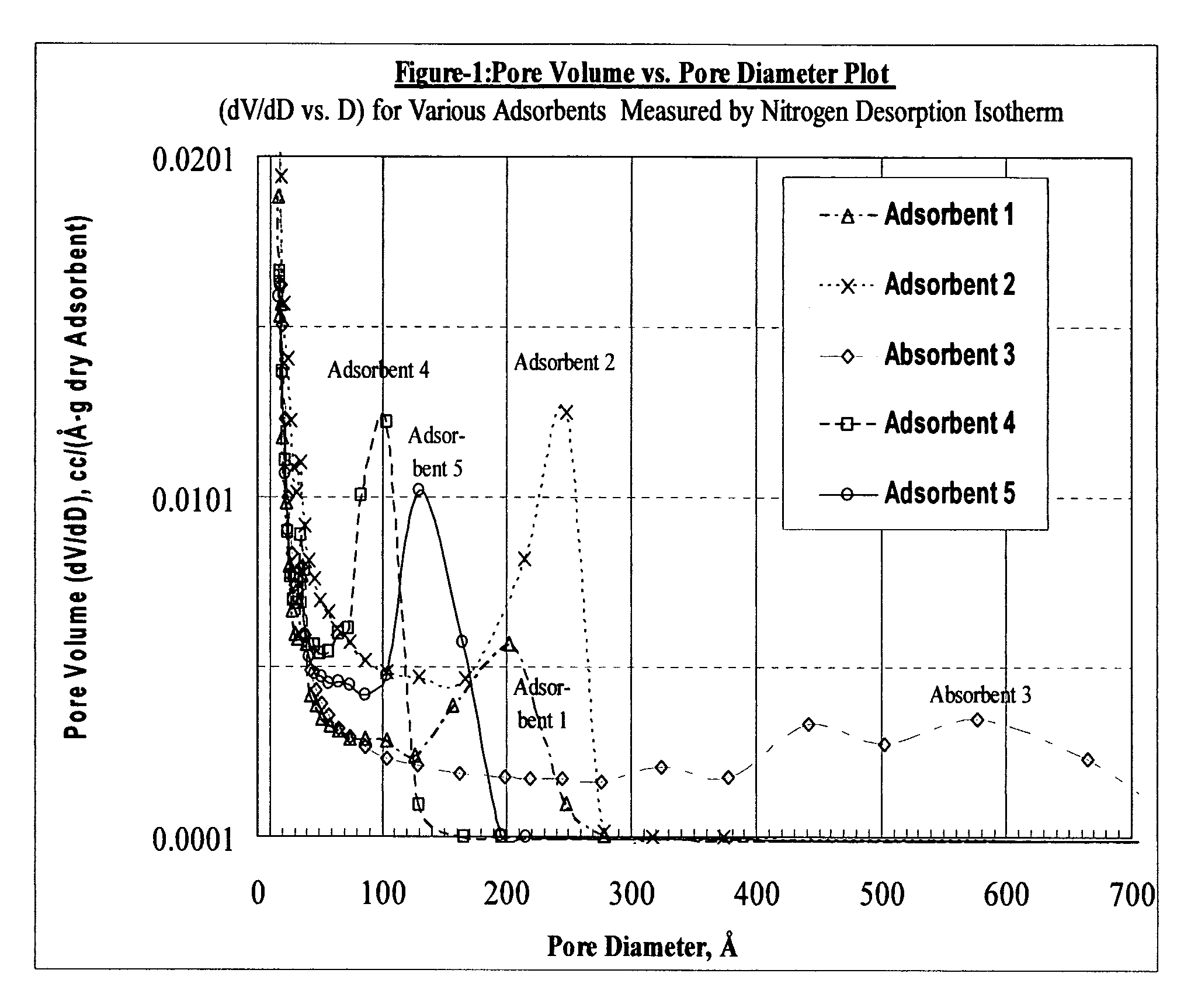
Size-selective hemocompatible porous polymeric adsorbents are provided with a pore structure capable of excluding molecules larger than 50,000 Daltons, but with a pore system that allows good ingress and egress of molecules smaller than 35,000 Daltons. The pore system in these porous polymeric adsorbents is controlled by the method of synthesis so that 98% of the total pore volume is located in pores smaller than 300 Angstroms (Å) in diameter with a working pore size range within 100 to 300 Å in diameter. The porous polymeric adsorbents of this invention are very selective for extracting midsize proteins, such as cytokines and β2-microglobulin, from blood and other physiologic fluids while keeping the components required for good health such as cells, platelets, albumin, hemoglobin, fibrinogen, and other serum proteins intact.
Owner:CYTOSORBENTS CORP
Preparation method of nano-particle@minisize metal organic frame material
InactiveCN107349964AAvoid reunionShort diffusion pathMaterial nanotechnologyOrganic-compounds/hydrides/coordination-complexes catalystsMetal-organic frameworkBiological imaging
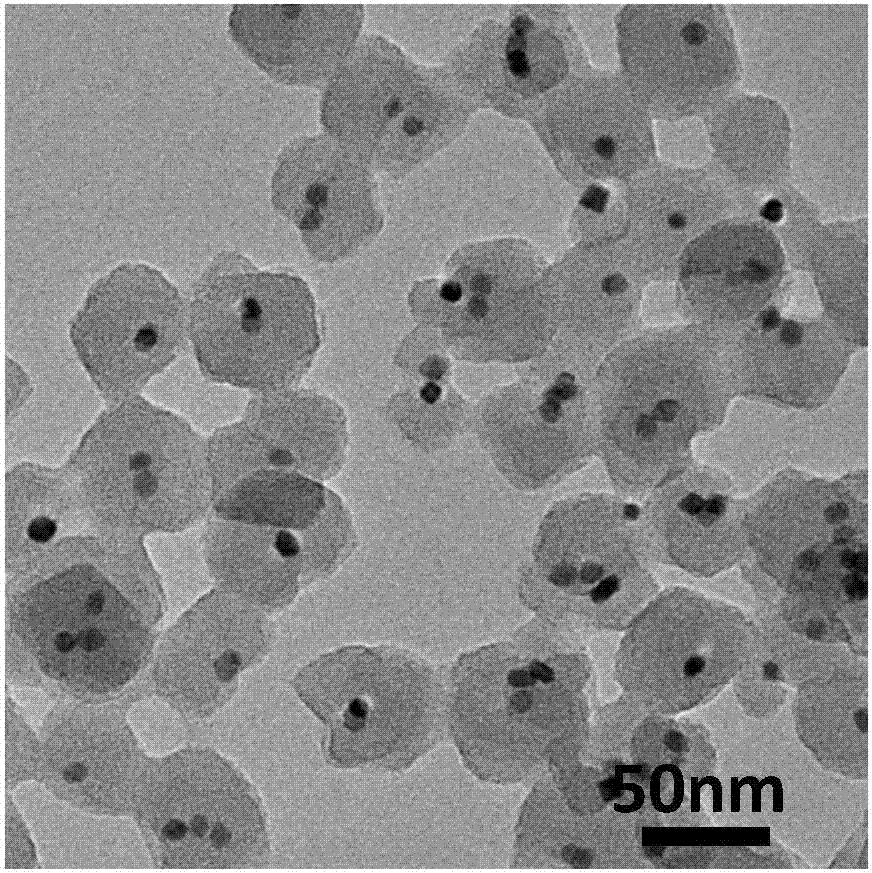
The invention relates to a preparation method of a nano-particle@minisize metal organic frame material. The preparation method comprises the following steps of 1, preparing nano-particles modified by polyvinylpyrrolidone; 2, adding the obtained nano-particles into an MOF growth reaction solution, the addition amount of an adjusting agent is controlled, and the nano-particle@minisize metal organic frame material is obtained. The method is simple and easy to implement, mild in condition and high in applicability, and the prepared nano-particle@minisize metal organic frame material can effectively prevent nano-particle aggregation, meanwhile has the advantages of being good in size selectivity, and short in diffusion range between the surface of the metal organic framework and the active center of nano-particles, and has the wide application prospect in the fields of catalysis, biological imaging and the like.
Owner:BEIJING UNIV OF CHEM TECH
Membranes with embedded nanotubes for selective permeability
ActiveUS7993524B2Improve permeabilityHigh selectivityCeramic shaping apparatusReverse osmosisFiltrationNanometre
Membranes for filtration by size exclusion are formed from open-ended nanotubes embedded in a polymeric matrix. The matrix forms a layer whose thickness is substantially less than the average length of the nanotubes, allowing the nanotubes to be randomly oriented throughout the matrix while providing channels extending through the layer for the selective passage of molecular species or particles based on size.
Owner:NAGARE MEMBRANES
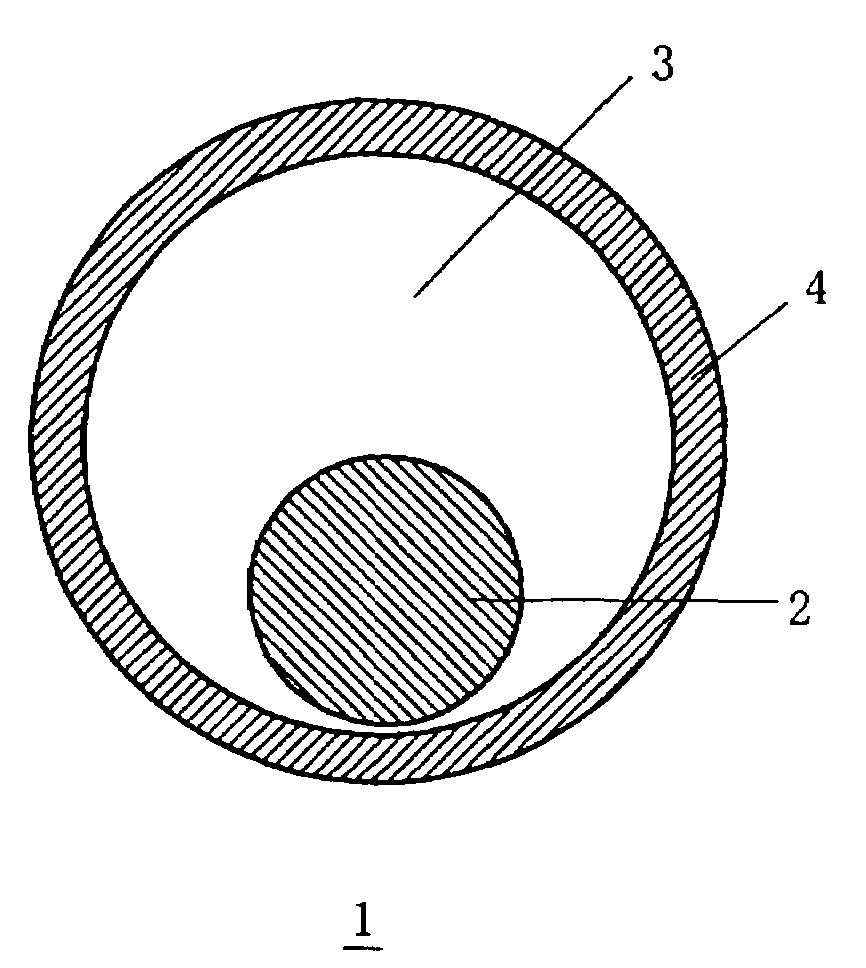
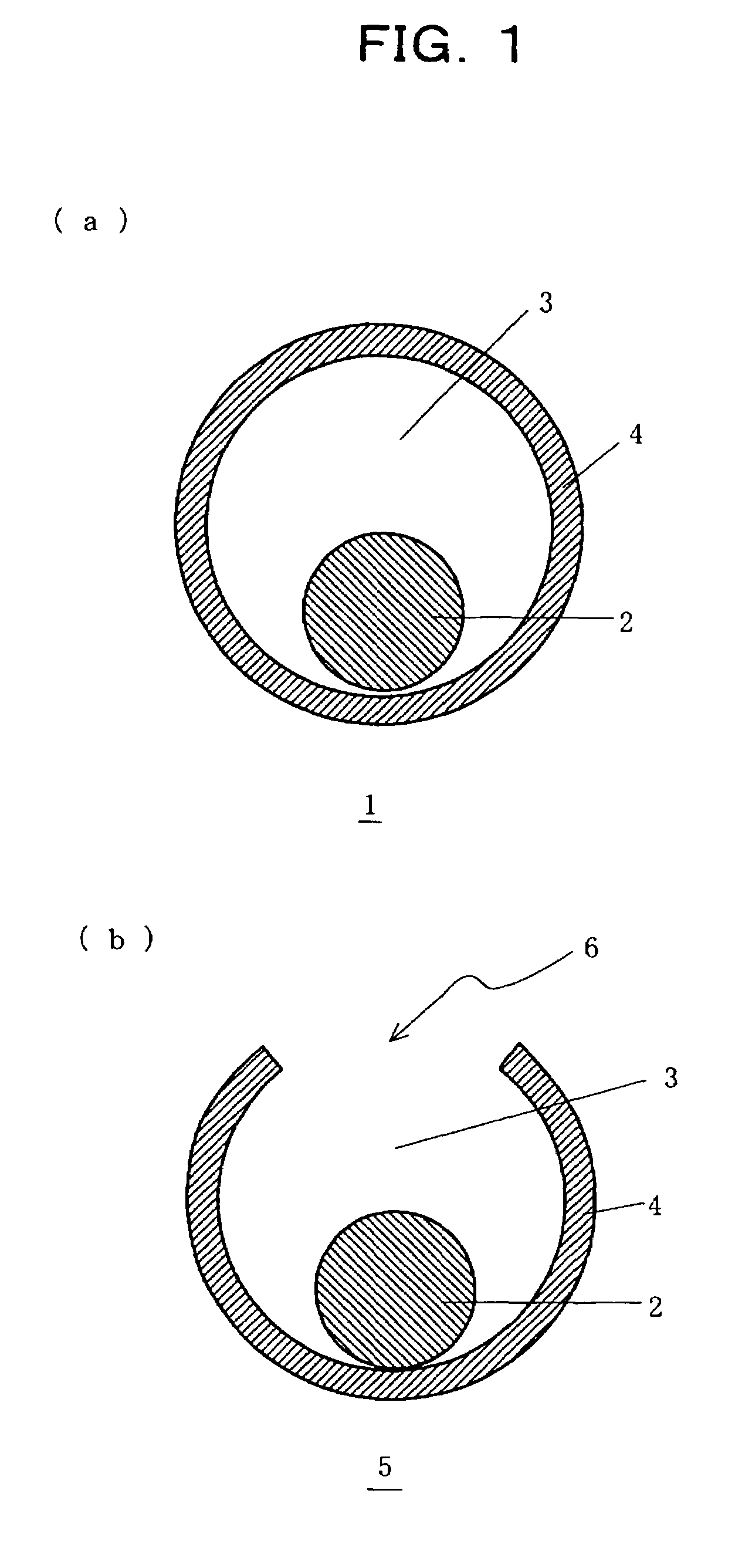
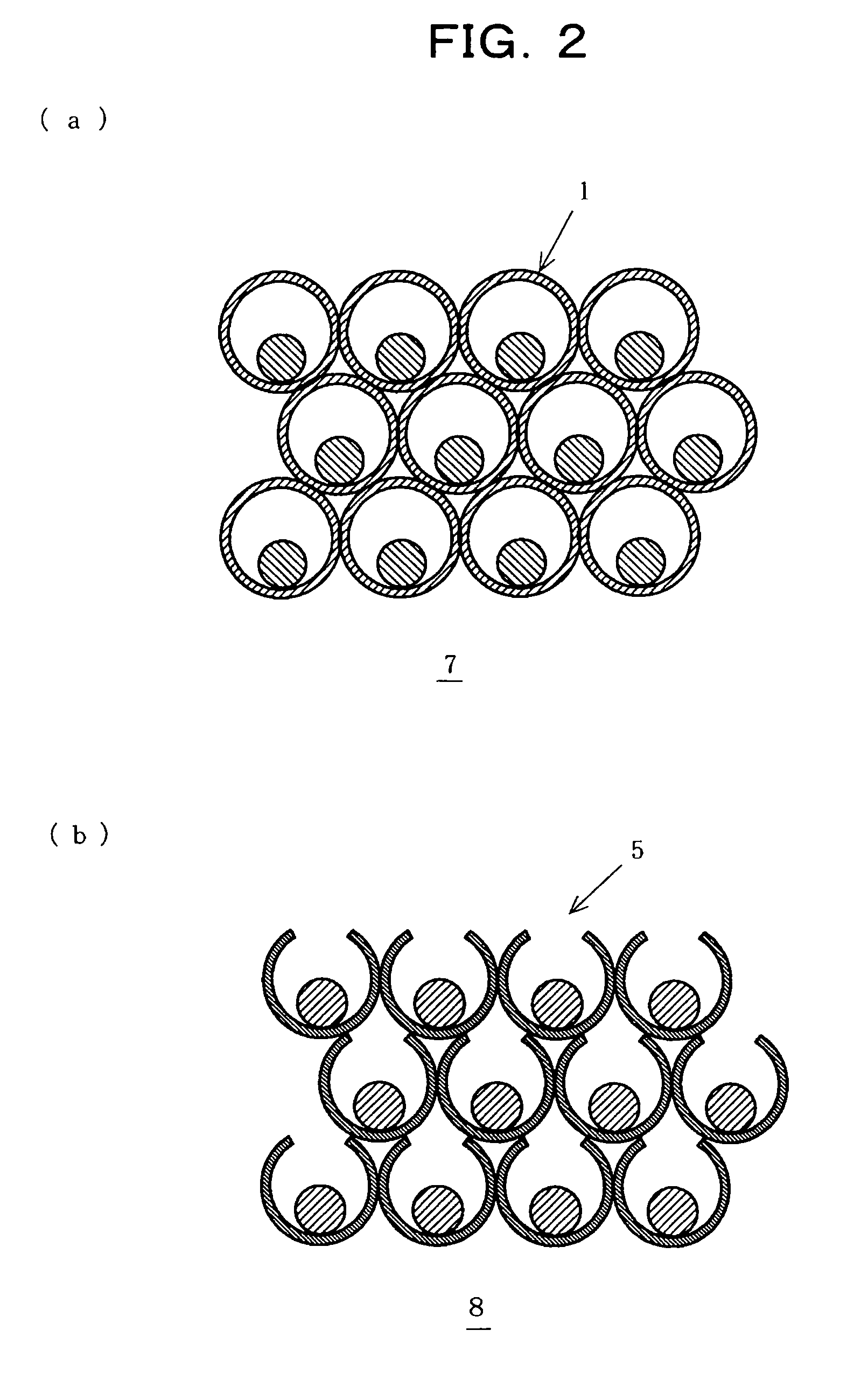
Core-shell structure having controlled cavity inside and structure comprising the core-shell structure as component, and method for preparation thereof
InactiveUS7381465B2Reduce the overall diameterMaterial nanotechnologyLiquid surface applicatorsNanoparticleSize selective
A core-shell structure comprises a core (2) comprising nanoparticles and a shell (4) coating the core (2), and its void space (3) formed by the core (2) and the shell (4) is controlled. A method of preparing the core-shell structure comprises: forming particles comprising a photoetchable semiconductor, metal or polymer and coating the particles with a shell (4) comprising a non-photoetchable semiconductor, metal or polymer, to form a core-shell structure (5); and irradiating the core-shell structure with a light having a controlled wavelength in the photoetching solution to form an adjustable void space inside a shell (3) within the core-shell structure by the size-selective photoetching method. The core-shell structure allows the preparation of a catalyst exhibiting an extremely high efficiency, and can be used as a precursor for preparing a nanomaterial required for a nanodevice.
Owner:JAPAN SCI & TECH CORP
Preparation method of layered molybdenum sulfide nanosheet molecular separating membrane
InactiveCN103585896ALow costIncrease productionSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisMaterials preparationFiltration
The invention discloses a preparation method of a layered molybdenum sulfide nanosheet molecule separating membrane. According to the preparation method, n-butyllithium is taken as an intercalation modular and enters a molybdenum sulfide crystal lattice after long-time stirring; water is added for decomposing n-butyllithium, so that the interlayer distances of a molybdenum sulfide sheet are enlarged; molybdenum sulfide is dispersed into monoatomic layer materials through ultrasonic treatment; redundant impurities are removed through dialysis, and a purified and clean molybdenum sulfide water solution is obtained; the obtained molybdenum sulfide water solution is treated with a vacuum filtration method, and the layered molybdenum sulfide nanosheet molecule separating membrane is obtained; and the thickness of the membrane is adjusted by adjusting the volume of the used molybdenum sulfide water solution. A molecule-selective separation membrane is obtained with a method of stacking the monoatomic layer materials to form the membrane; and the material preparation method is simple, the cost is low, and the obtained membrane has size selective separation performance on different molecules.
Owner:ZHEJIANG UNIV
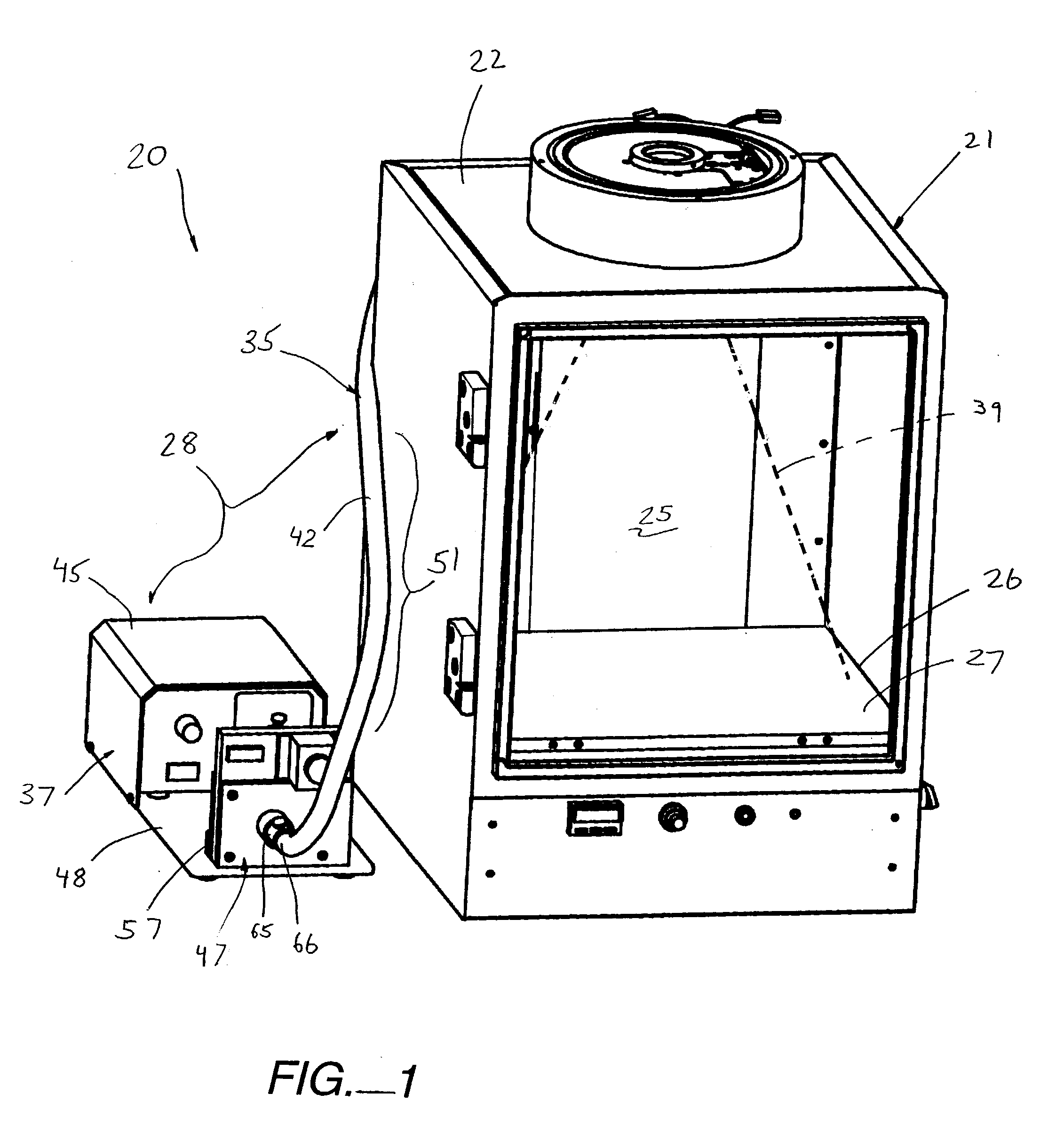
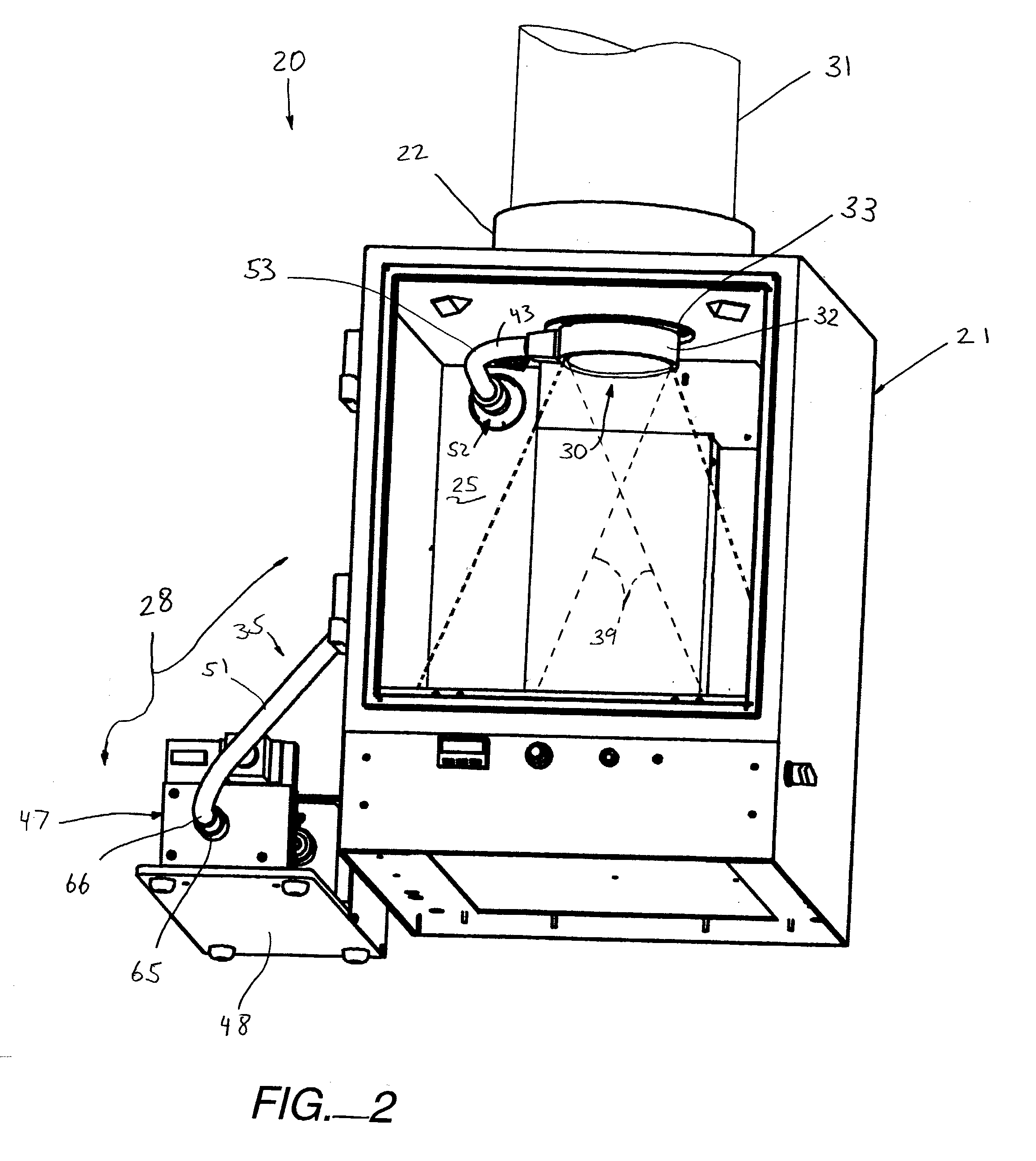
Bottom fluorescence illumination assembly for an imaging apparatus
Owner:XENOGEN CORP
Bottom fluorescence illumination assembly for an imaging apparatus
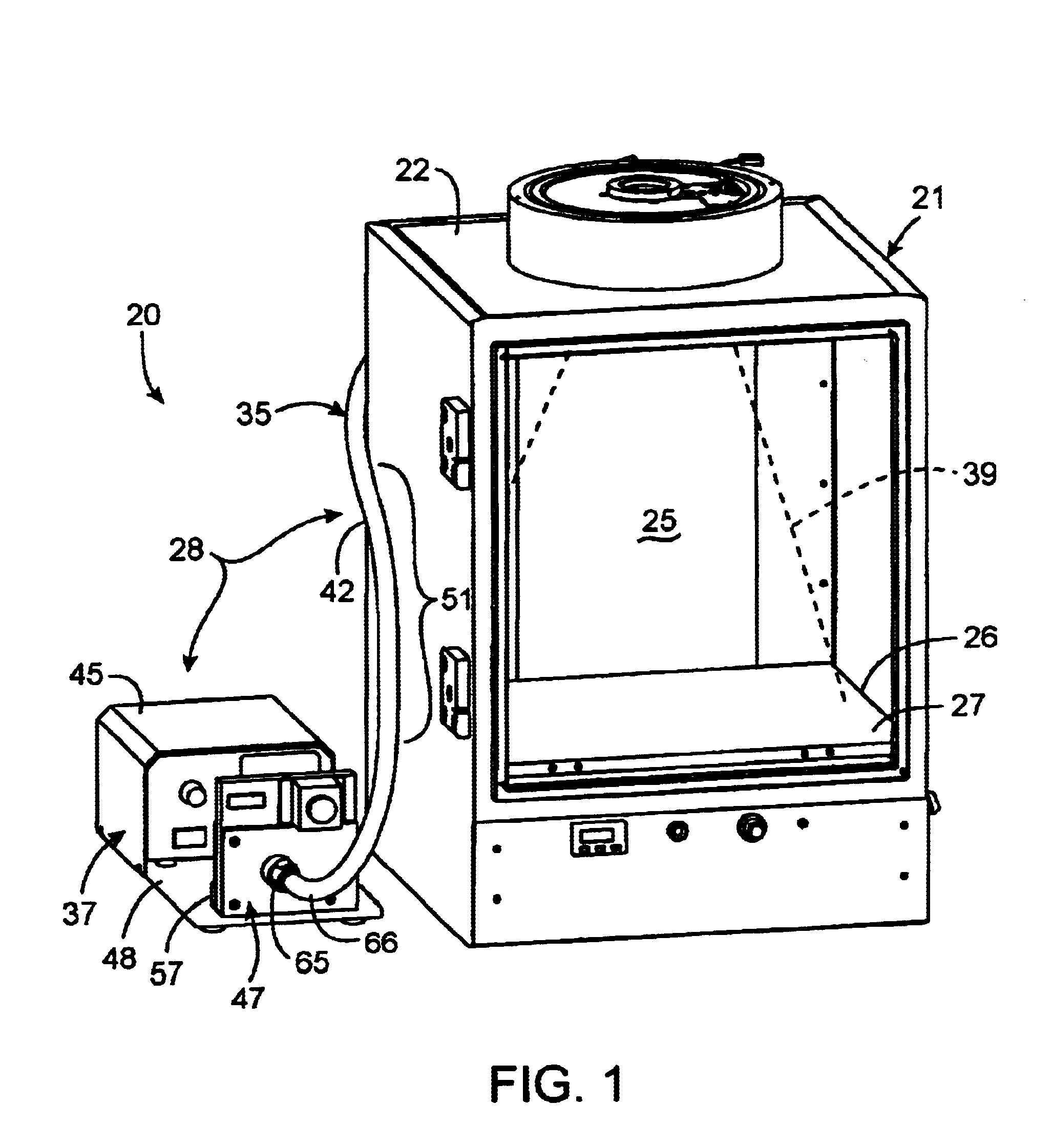
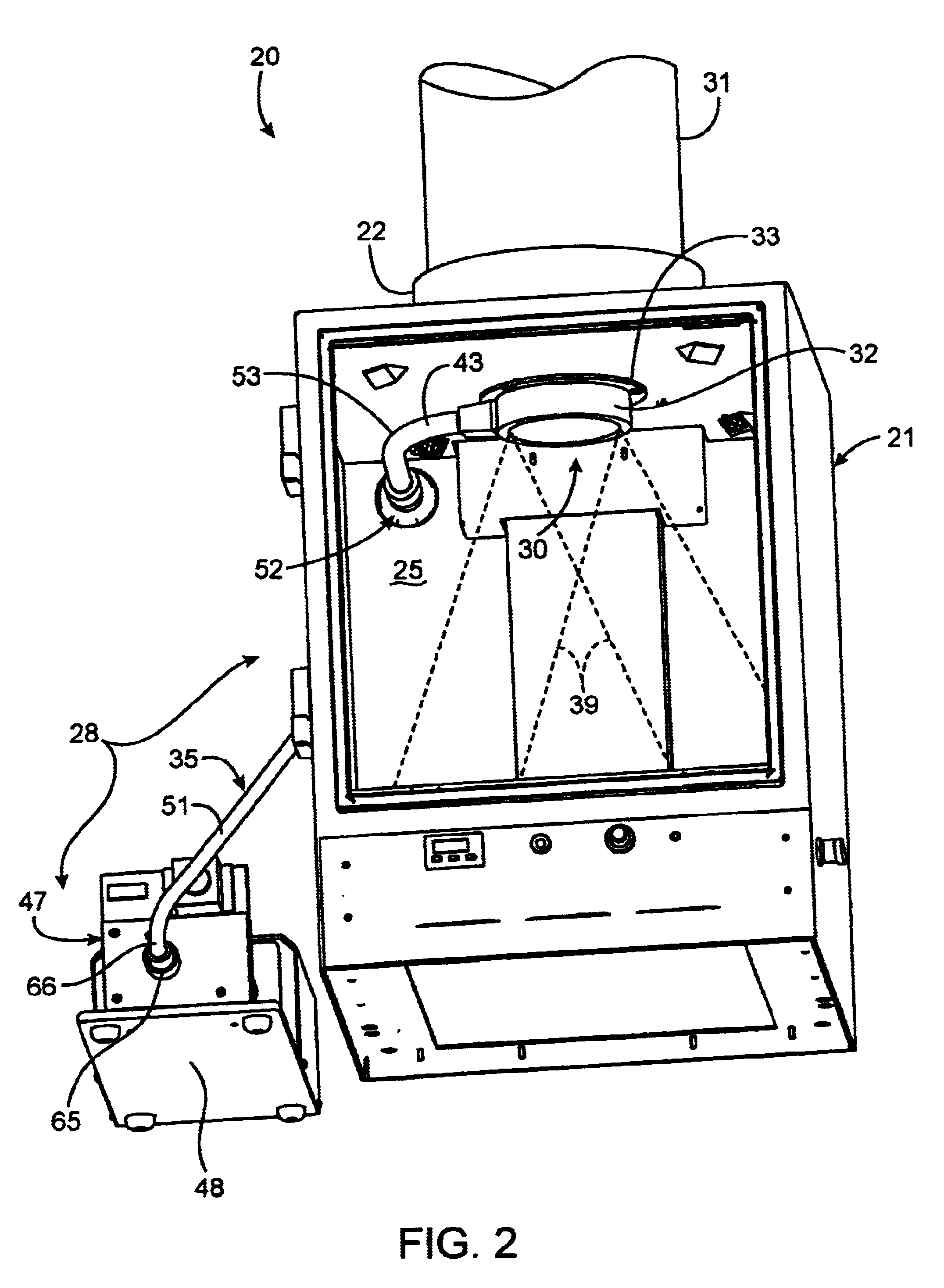
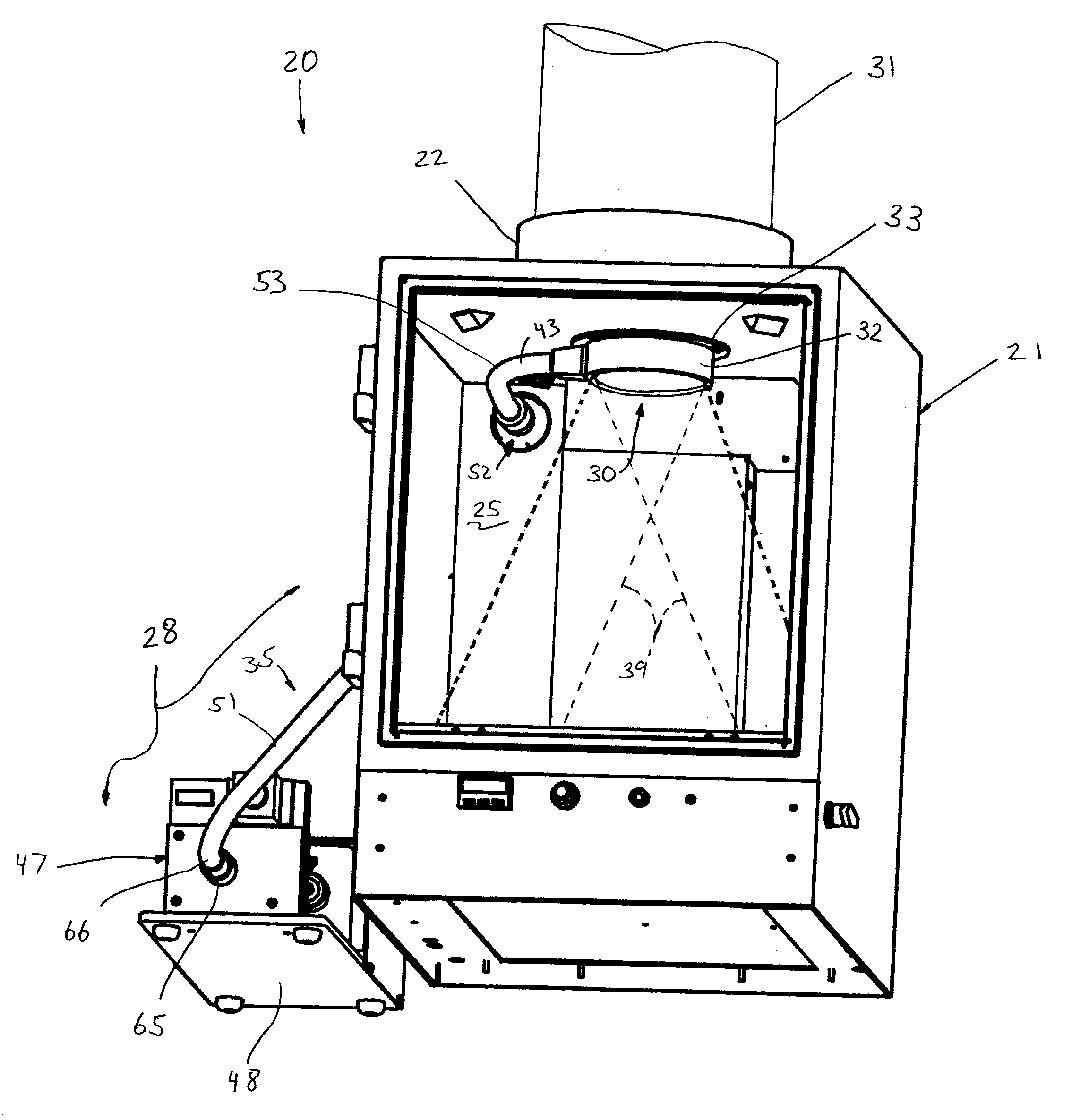
InactiveUS20030187344A1Reduce signalingSpectrum investigationLuminescent dosimetersFiberFluorescence
A macroscopic fluorescence illumination assembly is provided for use with an imaging apparatus with a light-tight imaging compartment. The imaging apparatus includes an interior wall defining a view port extending into the imaging compartment to enable viewing of a specimen contained therein. The illumination assembly includes a specimen support surface sized and dimensioned for receipt in the imaging compartment, and oriented to face toward the view port of the imaging apparatus. The support surface is substantially opaque and defines a window portion that enables the passage of light there through. The window portion is selectively sized and dimensioned such that the specimen, when supported atop the support surface, can be positioned and seated over the window portion in a manner forming a light-tight seal substantially there between. The illumination assembly further includes an excitation light source, and a bundle of fiber optic strands having proximal ends thereof in optical communication with the light source. The distal ends of the strands terminate proximate the window portion of the support surface. The distal ends each emit a respective beam of light originating from the light source which are then collectively directed toward the window portion and into a bottom side of the specimen wherein the diffused light passes there through and exits a topside thereof for receipt through the view port to view the fluorescence of the specimen.
Owner:XENOGEN CORP
Preparation method for mesoporous compound film
ActiveCN106731886AMesopore size is continuously adjustableUnique mesopore sizeSemi-permeable membranesOther chemical processesPolyethylene oxideUltrafiltration
The invention provides a preparation method for a mesoporous compound film. The method comprises the following steps: triggering the alcoholysis reaction of a precursor compound and a structure-directing agent in ethyl alcohol, thereby obtaining a sol; performing surface treatment on a porous film and soaking in the sol; airing the soaked porous film under a room temperature condition and completing a sol-gelling process; cleaning and drying, thereby obtaining the mesoporous compound film. According to the invention, a polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer, cetyl trimethyl ammonium bromide or lauryl sodium sulfate is taken as the structure-directing agent, so that the mesoporous size of the prepared mesoporous compound film can be continuously adjusted. The mesoporous compound film prepared according to the invention has a unique mesoporous structure; due to the organic solvent resistance and special porous structure or the capability of further modifying the mesoporous of the compound film, the mesoporous compound film can be applied to the separating purification fields of selective molecule transferring in the solution, the size selective ion transferring and ultrafiltration on a separating and liquid / liquid interface, and the like; the preparation process is simple; the method is environment-friendly.
Owner:SHENZHEN SENIOR TECH MATERIAL
Connecting system with direct plug connection
ActiveUS7568939B2Selectively usedSmall diameterCoupling device connectionsContact members penetrating/cutting insulation/cable strandsElectrical conductorEngineering
A plug-in connector arrangement includes a terminal block containing a chamber in which are mounted a horizontal bus bar having a transverse wall, and a resilient contact having a fixed horizontal leg portion, an intermediate portion bent upwardly from the first leg portion, and an outwardly biased second leg portion reversely bent back above the first leg portion. The second leg portion contains a clamping end portion that extends through a conductor opening contained in the first leg portion. The clamping portion includes at least two discrete clamping surfaces so arranged that when a bare conductor is inserted into the conductor opening, the conductor circumferential surface is selectively engaged by one or more of the clamping surfaces in accordance with the diametrical size of the conductor. The conductor is biased by the clamping portion toward electrical engagement with the bus bar transverse wall.
Owner:WEIDMULLER INTERFACE GMBH & CO KG
Mobile phone holder for vehicle
ActiveUS9473607B2Good lookingBatteries circuit arrangementsVehicle componentsEngineeringMobile phone
Owner:HYUNDAI MOTOR CO LTD +1
Nanoparticles and production thereof
InactiveUS20070065665A1Good repeatabilityReduce variation in propertyMaterial nanotechnologySemiconductor/solid-state device manufacturingOptical propertyNanoparticle
Owner:HITACHI LTD
Size-selective polymer system
ActiveUS20110070424A1Easy to transportMaterial nanotechnologySemi-permeable membranesPolymer sciencePolymer
A size-selective hemocompatible porous polymeric adsorbent system is provided, the polymer system comprises at least one polymer with a plurality of pores, and the polymer has at least one transport pore with a diameter from about 250 Angstroms to about 2000 Angstroms, and the polymer has a transport pore volume greater than about 1.8% to about 78% of a capacity pore of volume of the polymer.
Owner:CYTOSORBENTS CORP
Size-selective polymer system
ActiveUS8211310B2Easy to transportMaterial nanotechnologySemi-permeable membranesPolymer scienceSize selective
A size-selective hemocompatible porous polymeric adsorbent system is provided, the polymer system comprises at least one polymer with a plurality of pores, and the polymer has at least one transport pore with a diameter from about 250 Angstroms to about 2000 Angstroms, and the polymer has a transport pore volume greater than about 1.8% to about 78% of a capacity pore of volume of the polymer.
Owner:CYTOSORBENTS CORP
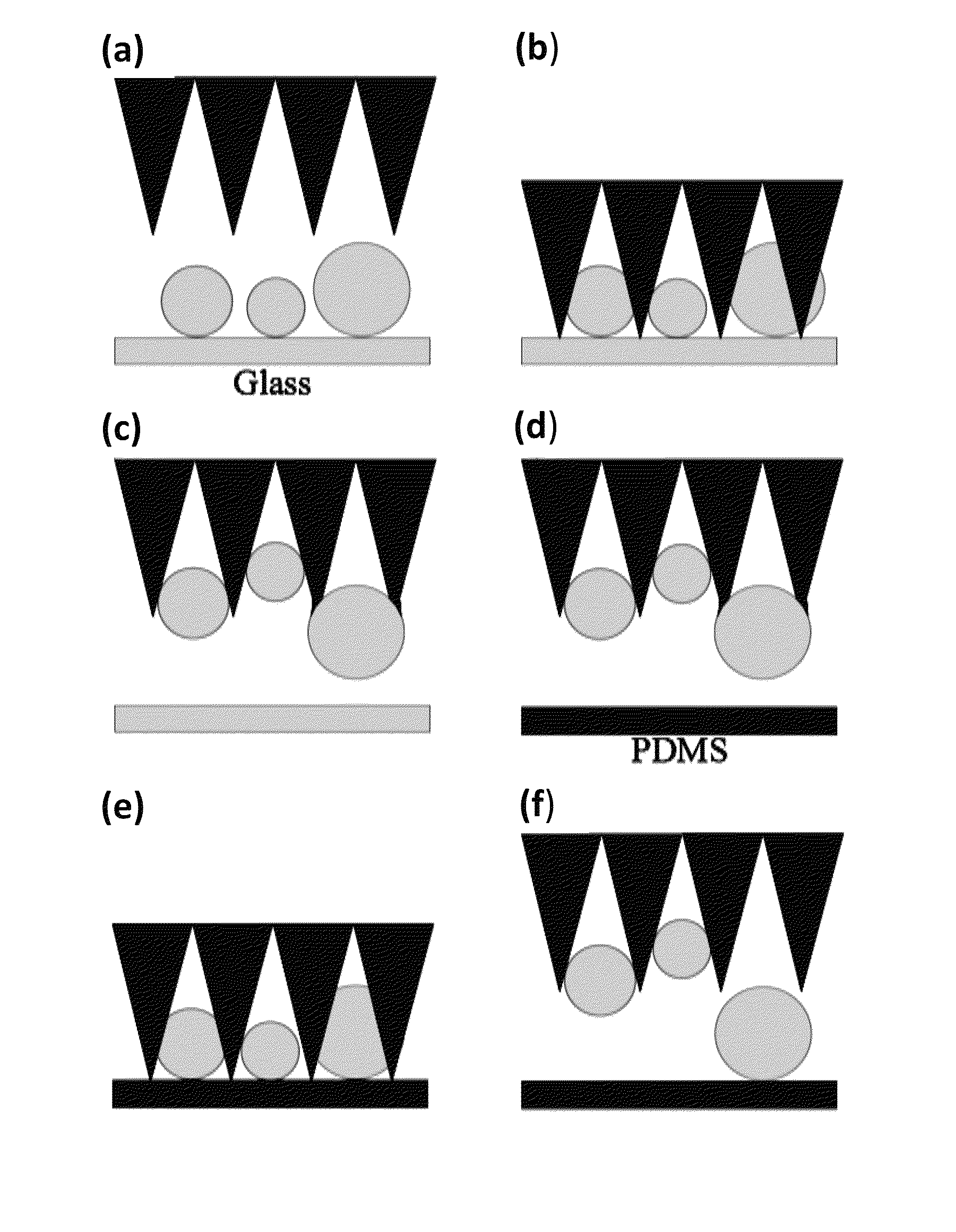
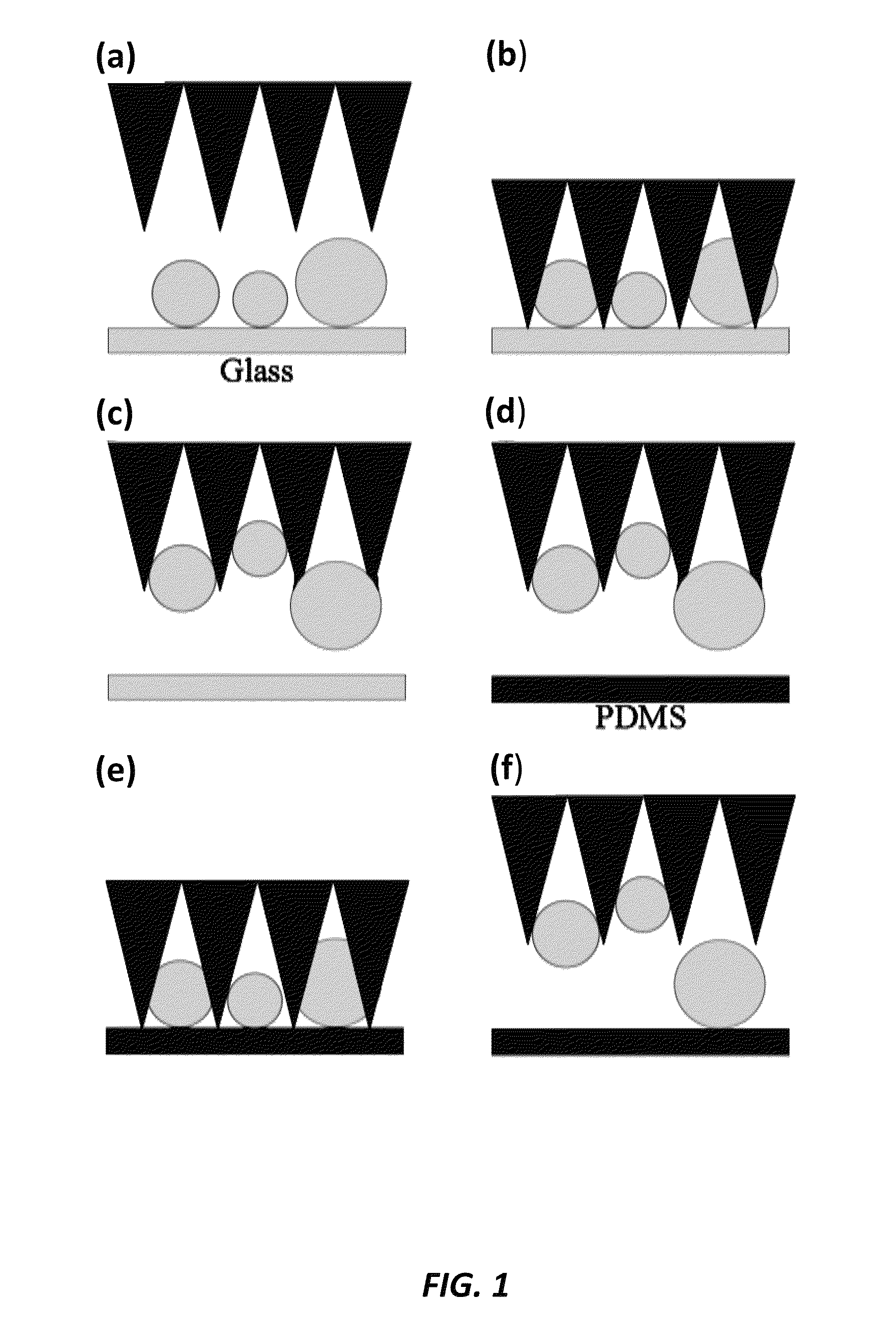
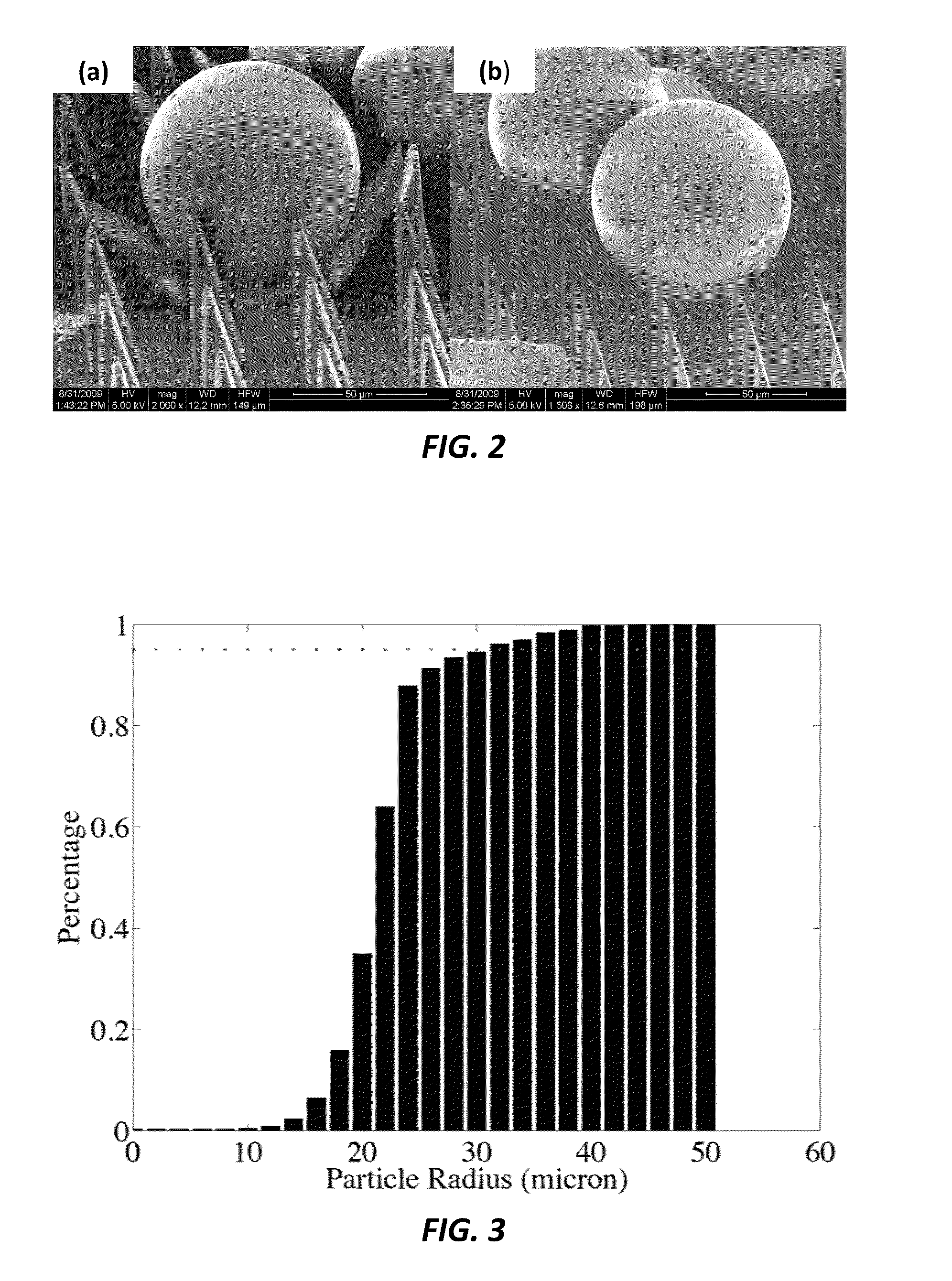
Micro-structure-based adhesives for size-selective particle trapping and sorting
InactiveUS8882996B2Nanostructure manipulationCleaning processes and apparatusParticle trappingMicro structure
A method of separating particles is provided that includes exposing a selective PDMS adhesive to a particle-contaminated surface, where the selective PDMS adhesive captures particles present on the particle-contaminated surface to form a fouled selective PDMS adhesive, and exposing the fouled selective PDMS adhesive to a PDMS transfer sheet, where particles outside of a desired range are transferred over to the PDMS transfer sheet, where the fouled selective PDMS adhesive retains only the particles of a desired range.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Insert for a child's seat
An insert for the seat of a child is provided. The insert has detachable elements which may be selectively added or changed depending on the size of the child. The insert has a head rest having a removable interior hollow sound channel or a two way traditional speaker system. The head rest has an internal Bluetooth, a micro SD / TF slot and / or an audio port which may connect to an external mp3 player. A plurality of parallel slits through the main body section of the insert allows harness hardware of a child's car seat (or other seat) to be inserted through the main body section of the insert at various locations; therein allowing the insert to be used in connection with various car seats.
Owner:THE BIRD & BEAR GRP LLC
Redox-Flow Batteries Employing Oligomeric Organic Active Materials and Size-Selective Microporous Polymer Membranes
Intermittent energy sources, including solar and wind, require scalable, low-cost, multi-hour energy storage solutions to be effectively incorporated into the grid. Redox-flow batteries offer a solution, but suffer from rapid capacity fade and low Coulombic efficiency due to the high permeability of redox-active species across the battery's membrane. Here we show that active-species crossover can be arrested by scaling the membrane's pore size to molecular dimensions and in turn increasing the size of the active material to be above the membrane's pore-size exclusion limit. When oligomeric redox-active organic molecules were paired with microporous polymer membranes, the rate of active-material crossover was either completely blocked or slowed more than 9,000-fold compared to traditional separators at minimal cost to ionic conductivity. In the case of the latter, this corresponds to an absolute rate of ROM crossover of less than 3 μmol cm−2 day−1 (for a 1.0 M concentration gradient), which exceeds performance targets recently set forth by the battery industry. This strategy was generalizable to both high and low-potential ROMs in a variety of electrolytes, highlighting the importance of macromolecular design in implementing next-generation redox-flow batteries.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
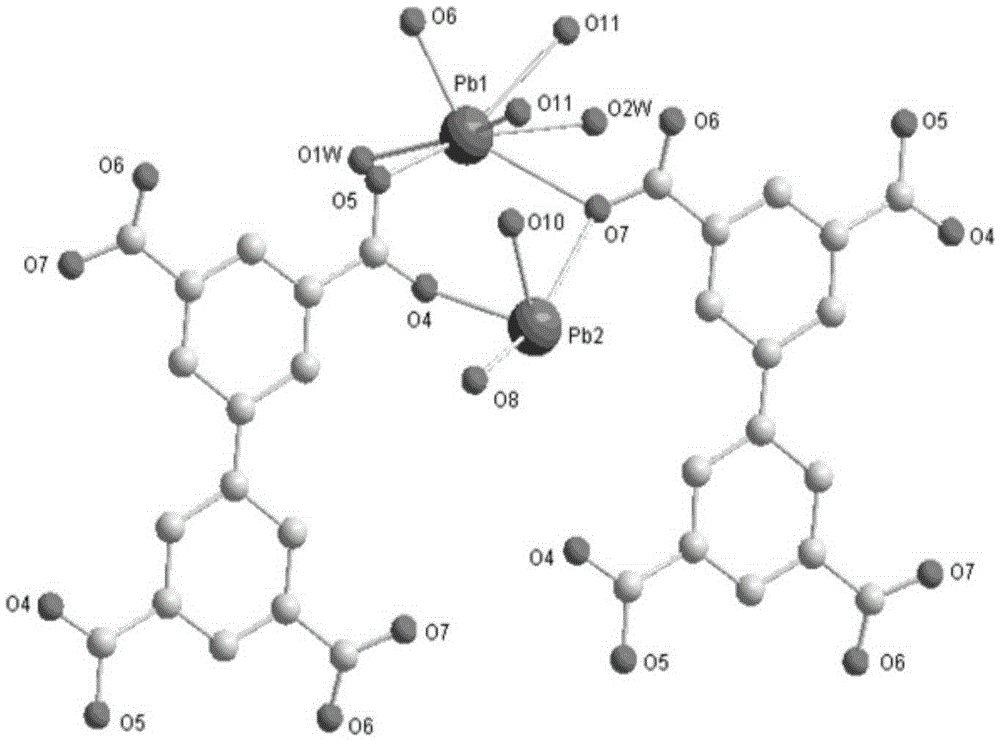
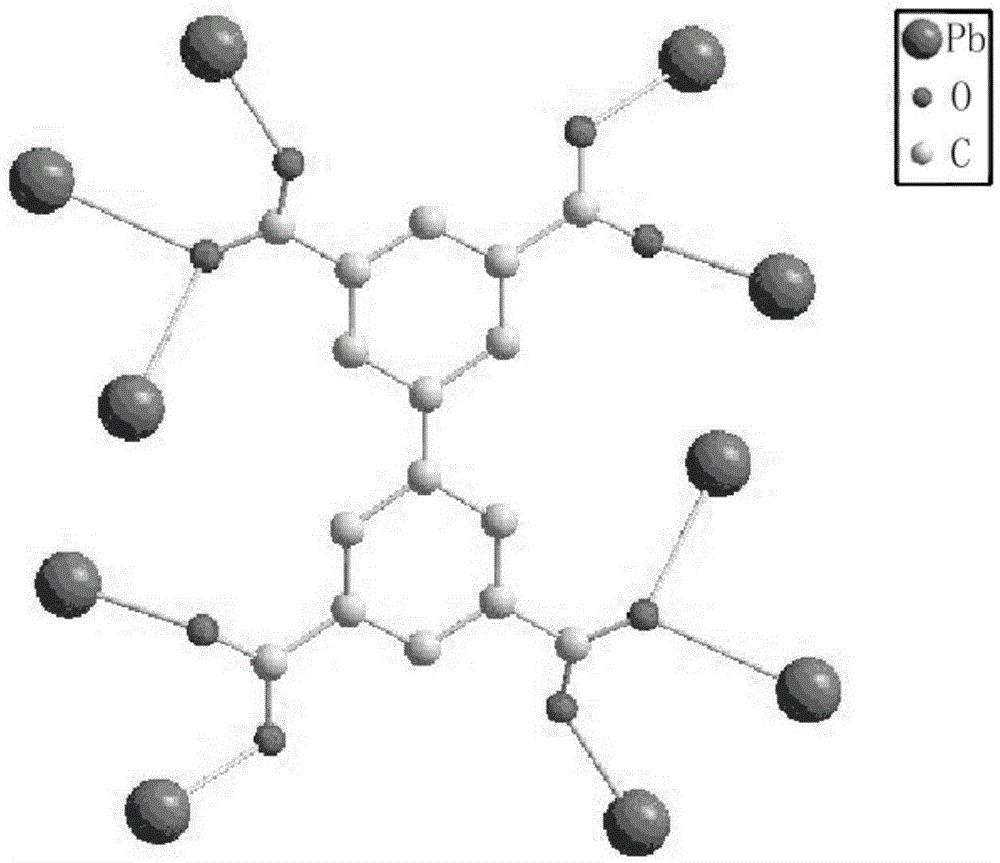
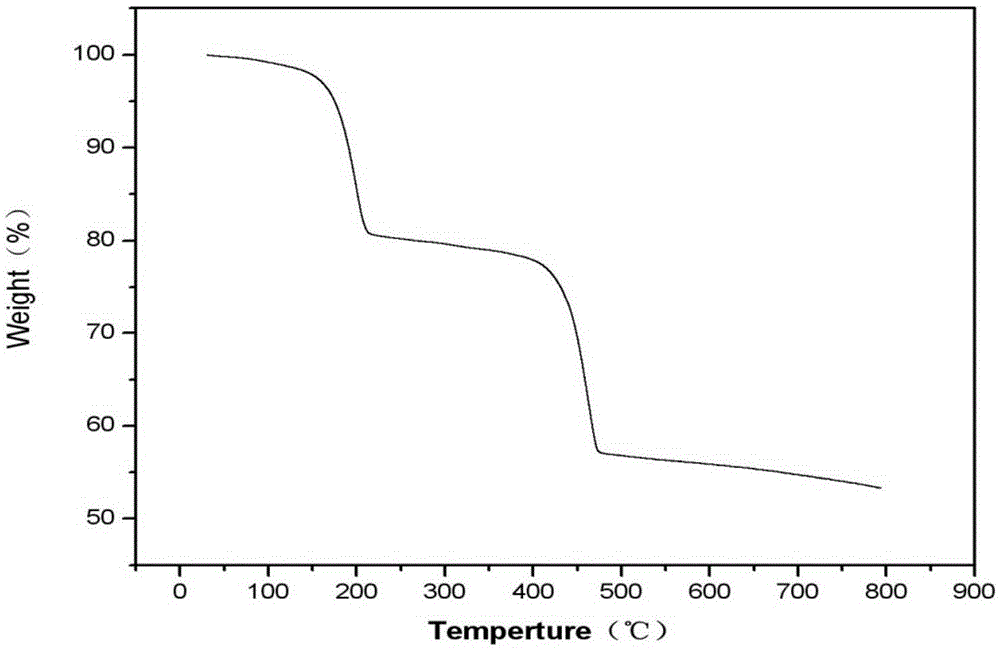
Metal organic framework material based on lead nitrate and application thereof
ActiveCN105237565AThree-dimensional pore structure is goodSatisfactory catalytic effectOrganic-compounds/hydrides/coordination-complexes catalystsLead organic compoundsLead nitrateMetal-organic framework
The invention relates to a metal organic framework material based on lead nitrate and application thereof. The technical scheme is that metal salt Pb(NO3)2, ligand H4BPTC and a solvent N,N-dimethylacetamide are placed in a container, ultrasonic oscillation is performed until the metal salt and the ligand are evenly scattered into the solvent; the solvent is sealed and then placed in an oven to stand for 72 h at 85 DEG C, white cube blocky crystal is obtained, washed, filtered and dried, and a target product is obtained. A method for preparing the metal organic framework material is simple, the metal organic framework material is stable in structure and has a good three-dimensional pore structure, the silicon cyanation can be catalyzed efficiently, reaction substrate aromatic aldehyde has size selectivity, and the catalytic reaction is performed on the anhydrous and oxygen-free conditions.
Owner:芜湖数字信息产业园有限公司
Size-selective hemocompatible polymer system
ActiveUS20120238441A1Easy to transportOther chemical processesSolid sorbent liquid separationSorbentSize selective
A size-selective hemocompatible porous polymeric adsorbent system is provided, the polymer system comprises at least one crosslinking agent and at least one dispersing agent, and the polymer has a plurality of pores with diameters in the range from about 17 to about 40,000 Angstroms
Owner:CYTOSORBENTS CORP
Method and apparatus for physical separation of different sized nanostructures
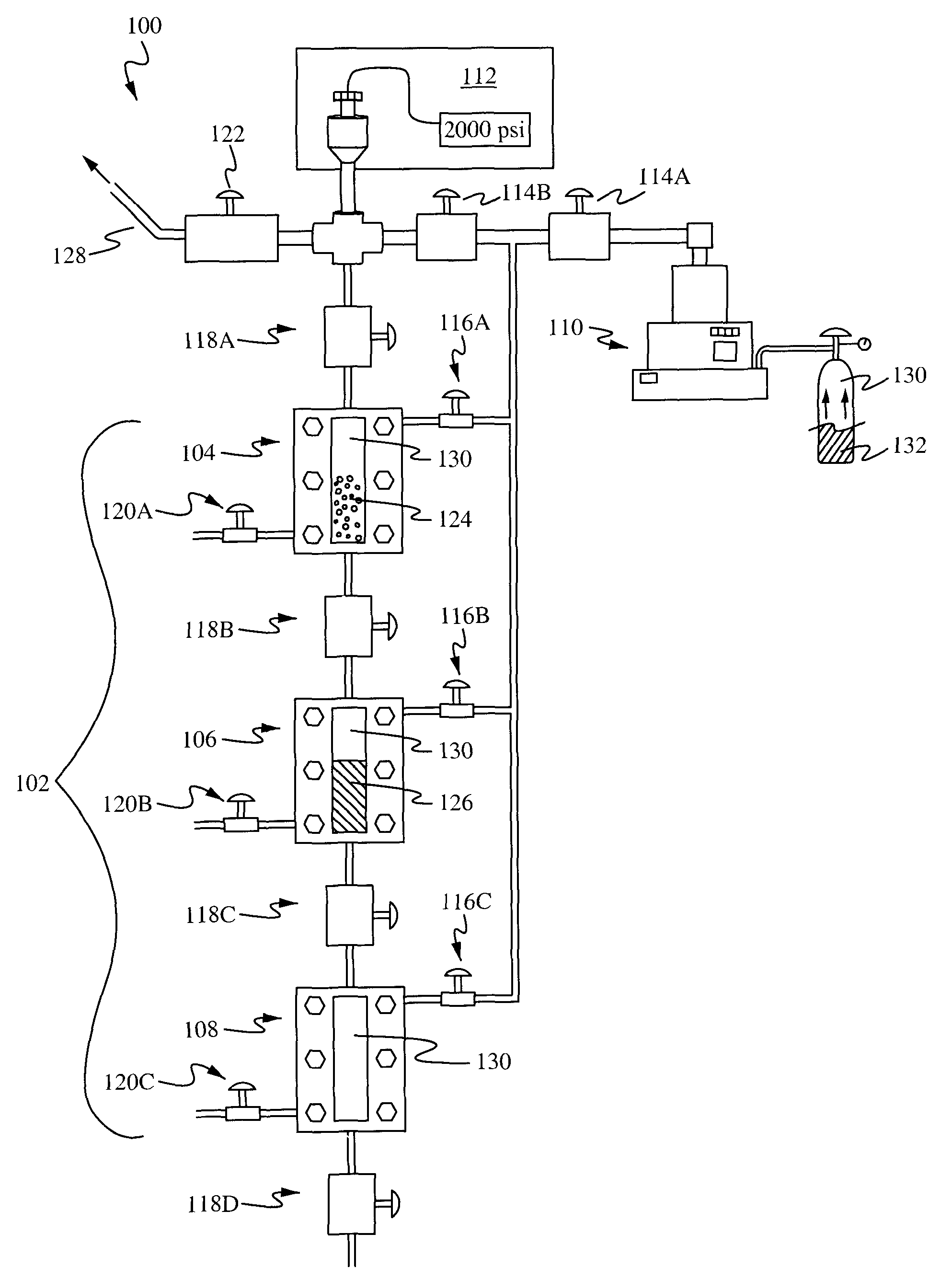
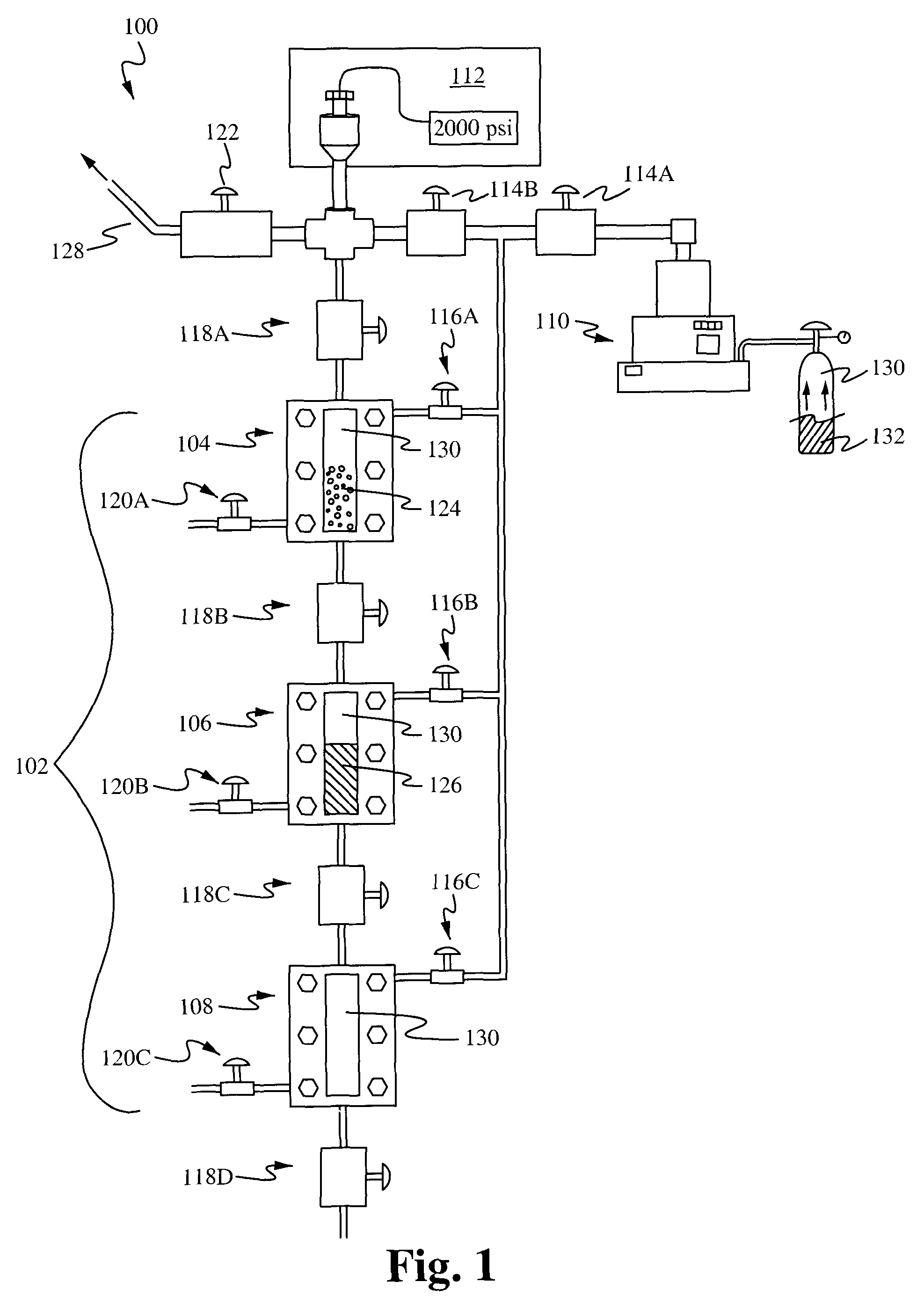
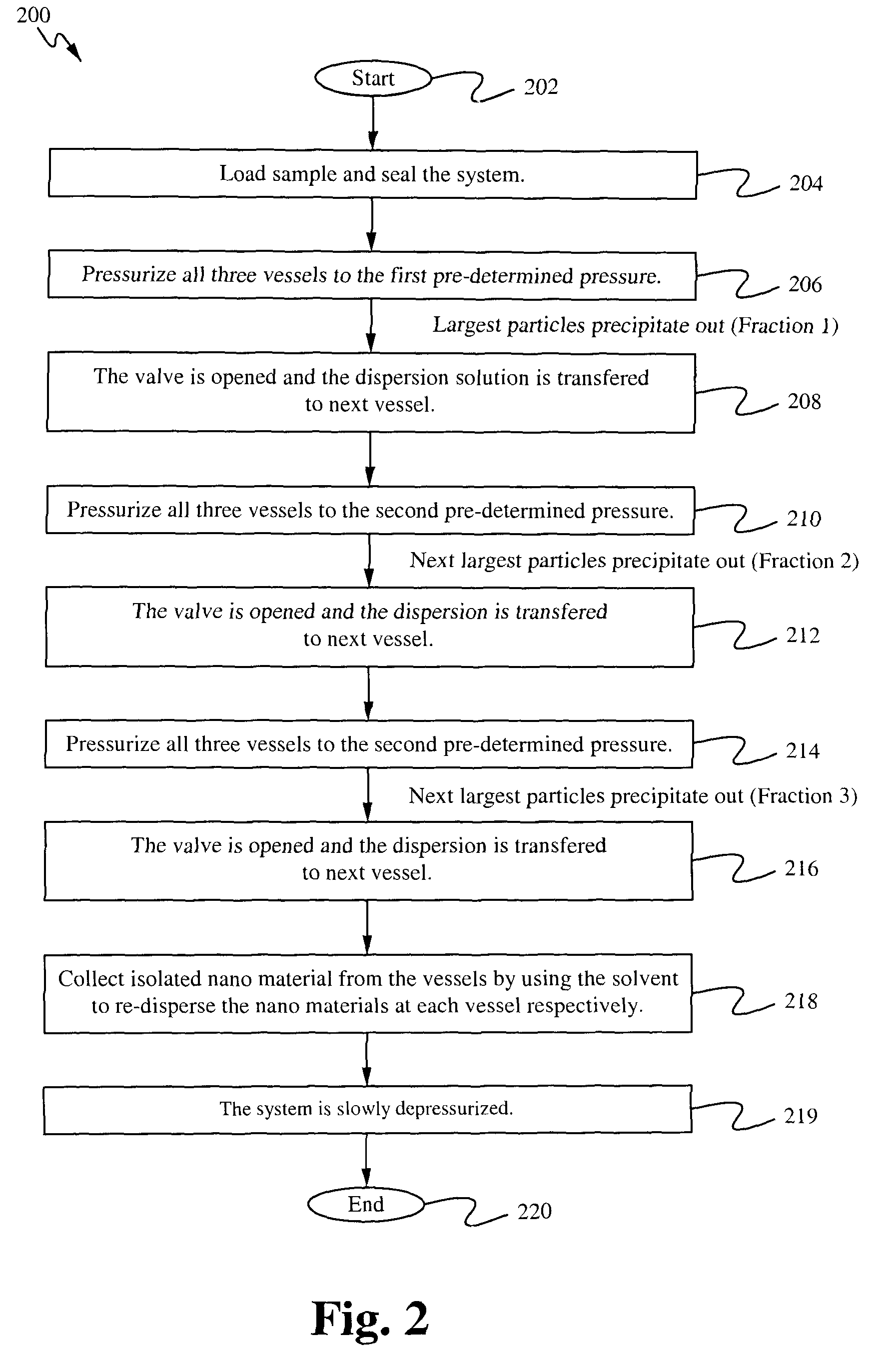
InactiveUS8215489B1Easy to adjustIncrease liquid volumeMaterial nanotechnologyReversed direction vortexFractionationSize selective
The present application provides apparatuses and methods for the size-selective fractionation of ligand-capped nanoparticles that utilizes the tunable thermophysical properties of gas-expanded liquids. The nanoparticle size separation processes are based on the controlled reduction of the solvent strength of an organic phase nanoparticle dispersion through increases in concentration of the antisolvent gas, such as CO2, via pressurization. The method of nanomaterial separation contains preparing a vessel having a solvent and dispersed nanoparticles, pressurizing the chamber with a gaseous antisolvent, and causing a first amount of the nanoparticles to precipitate, transporting the solution to a second vessel, pressurizing the second vessel with the gaseous antisolvent and causing further nanoparticles to separate from the solution.
Owner:AUBURN UNIV
Connecting system with direct plug connection
ActiveUS20090061669A1Selectively usedSmall diameterCoupling device connectionsClamped/spring connectionsElectrical conductorEngineering
A plug-in connector arrangement includes a terminal block containing a chamber in which are mounted a horizontal bus bar having a transverse wall, and a resilient contact having a fixed horizontal leg portion, an intermediate portion bent upwardly from the first leg portion, and an outwardly biased second leg portion reversely bent back above the first leg portion. The second leg portion contains a clamping end portion that extends through a conductor opening contained in the first leg portion. The clamping portion includes at least two discrete clamping surfaces so arranged that when a bare conductor is inserted into the conductor opening, the conductor circumferential surface is selectively engaged by one or more of the clamping surfaces in accordance with the diametrical size of the conductor. The conductor is biased by the clamping portion toward electrical engagement with the bus bar transverse wall.
Owner:WEIDMULLER INTERFACE GMBH & CO KG
Universal physiologic sampling pump (PSP) capable of rapid response to breathing
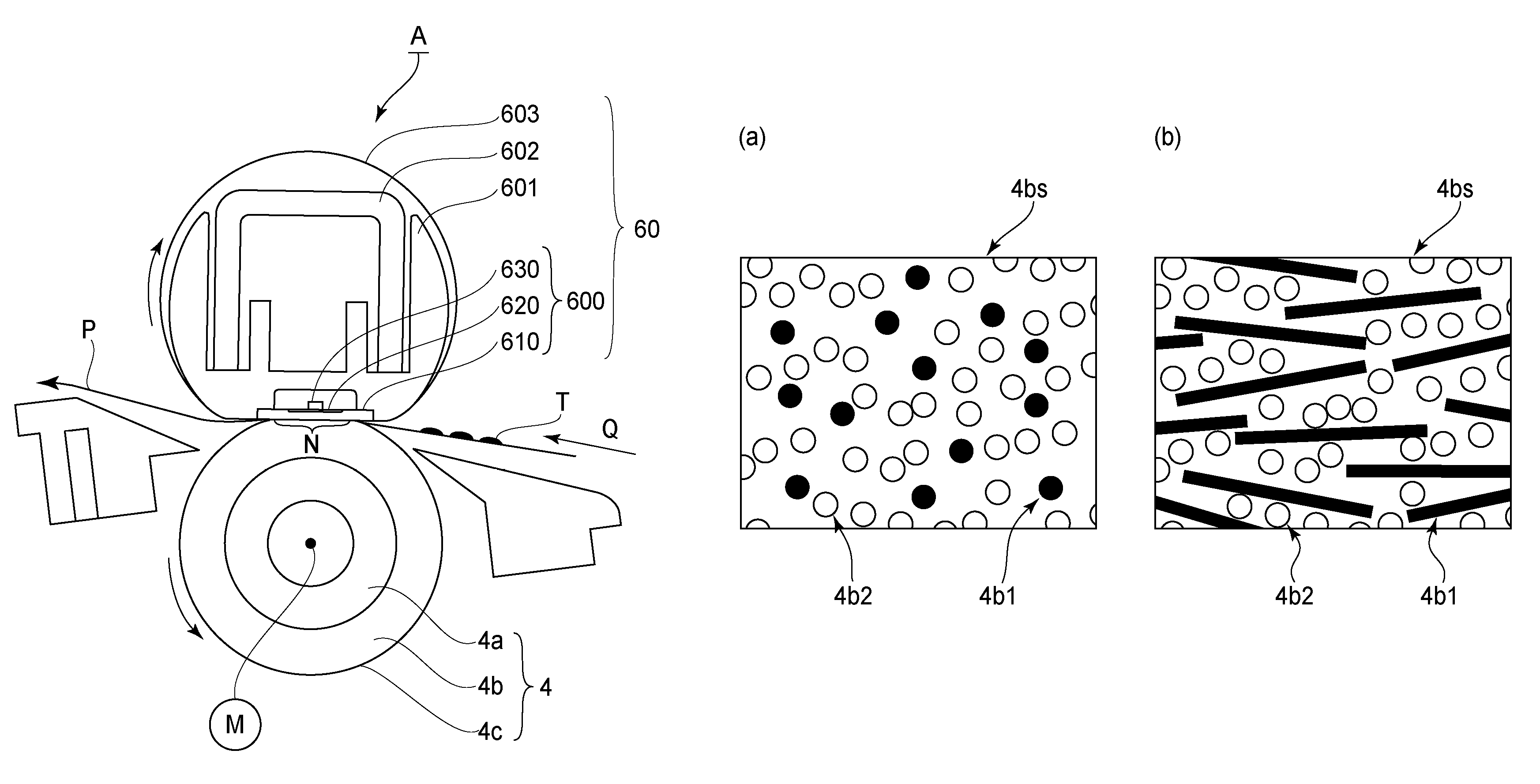
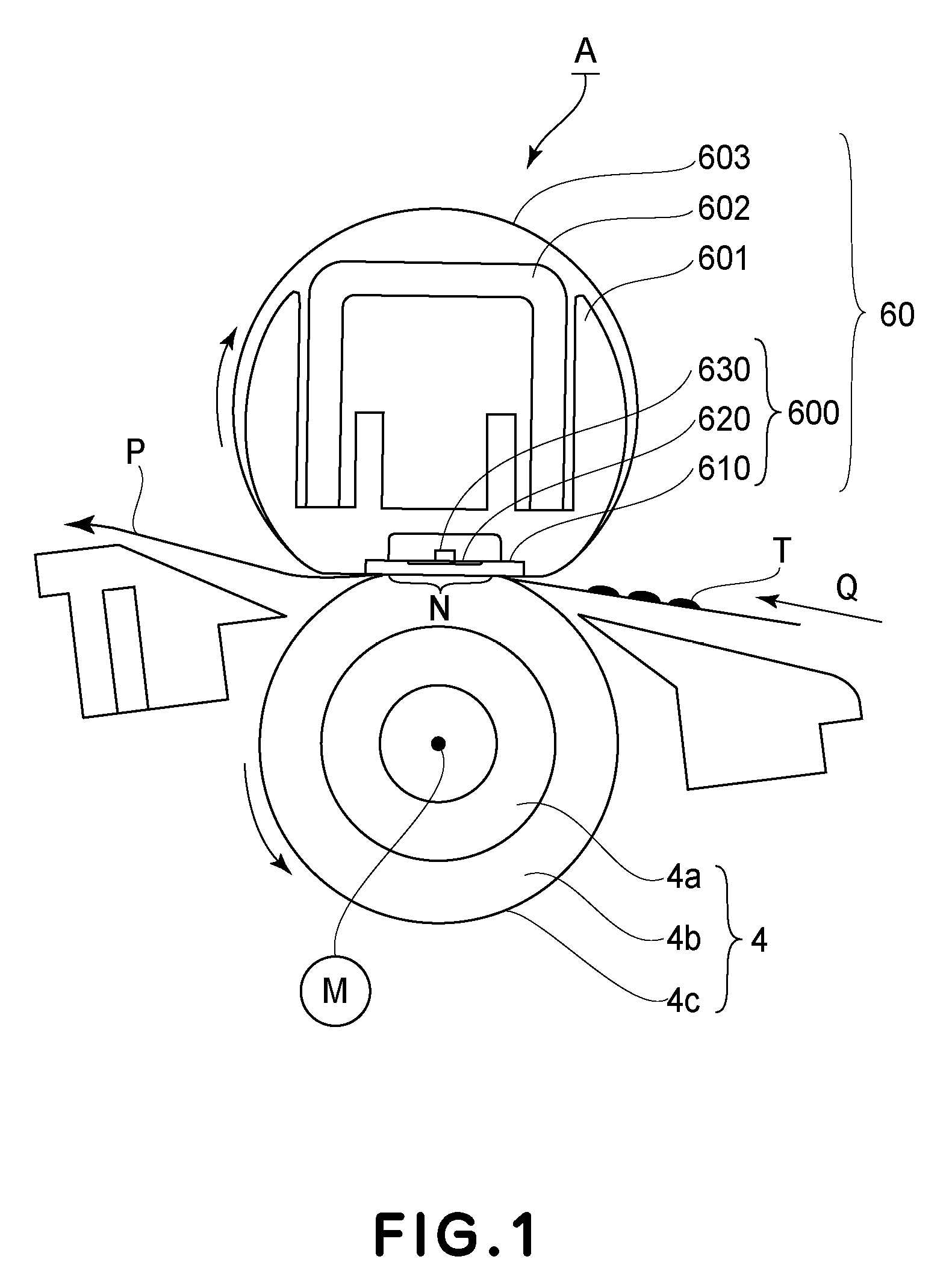
The present invention discloses a physiologic sampling pump (PSP) which uses at least one valve placed near the sampling medium to modulate air sampling to follow a person's inhalation rate and to obviate the sluggishness inherent in prior art PSPs caused by varying pump speed and by the propagation time through an air tube that connects the collection medium to prior art pumps thereby also obviating limitations inherent in system response, functionality, and accuracy. Moreover, by maintaining an essentially constant air flow through a cyclone at all times and through the collection medium while sampling, the present invention operates at known collection efficiencies, and is therefore capable of size-selective sampling of particulates as opposed to prior art PSPs that by varying the magnitude of air flow, make the separation efficiencies of pre-collection devices indeterminate and the samples worthless. When used instead with an impact sampling head, the present invention may collect total particulate as well, and may collect gases and vapors with a charcoal tube sampling head. Structural features associated with the physiological sampling pump for providing rapid response to breathing include an outer housing including a thereto-resistant case, multiple and interchangeable PSP sampling heads further including collection media and a valve(s) mounted on a valve manifold with associated tubing.
Owner:UNITED STATES OF AMERICA
Redox active polymers and colloidal particles for flow batteries
ActiveUS20160208030A1Reduce total powerReduce current densityRegenerative fuel cellsIndirect fuel cellsBulk electrolysisHigh concentration
The invention provides a redox flow battery comprising a microporous or nanoporous size-exclusion membrane, wherein one cell of the battery contains a redox-active polymer dissolved in the non-aqueous solvent or a redox-active colloidal particle dispersed in the non-aqueous solvent. The redox flow battery provides enhanced ionic conductivity across the electrolyte separator and reduced redox-active species crossover, thereby improving the performance and enabling widespread utilization. Redox active poly(vinylbenzyl ethylviologen) (RAPs) and redox active colloidal particles (RACs) were prepared and were found to be highly effective redox species. Controlled potential bulk electrolysis indicates that 94-99% of the nominal charge on different RAPs is accessible and the electrolysis products are stable upon cycling. The high concentration attainable (>2.0 M) for RAPs in common non-aqueous battery solvents, their electrochemical and chemical reversibility, and their hindered transport across porous separators make them attractive materials for non-aqueous redox flow batteries based on size-selectivity.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Size-selective hemoperfusion polymeric adsorbents
Size-selective hemocompatible porous polymeric adsorbents are provided with a pore structure capable of excluding molecules larger than 50,000 Daltons, but with a pore system that allows good ingress and egress of molecules smaller than 35,000 Daltons. The pore system in these porous polymeric adsorbents is controlled by the method of synthesis so that 98% of the total pore volume is located in pores smaller than 300 Angstroms (Å) in diameter with a working pore size range within 100 to 300 Å in diameter. The porous polymeric adsorbents of this invention are very selective for extracting midsize proteins, such as cytokines and β2-microglobulin, from blood and other physiologic fluids while keeping the components required for good health such as cells, platelets, albumin, hemoglobin, fibrinogen, and other serum proteins intact.
Owner:CYTOSORBENTS CORP
Hollow porous polymer nanospheres composite material encapsulated by noble metal nanoparticles, and synthesis and application thereof
InactiveCN110586182AHigh catalytic activityMany cyclesOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsSize selective
The invention discloses a synthesis method and size selective catalytic application of a hollow porous polymer nanosphere composite material encapsulated by noble metal nanoparticles. Firstly, a porous polymer nanosphere network material with a hollow structure is synthesized by a reversible addition-fracture chain transfer polymerization and friedel-crafts overcrosslinking one-pot reaction. Then,the material is used as a main carrier, various guest noble metal nanoparticles are encapsulated respectively into hollow cavities through an impregnation-reduction method, and the final hollow porous polymer nanosphere composite material encapsulated by the noble metal nanoparticles is formed. The synthesis method and size selective catalytic application of the hollow porous polymer nanosphere composite material encapsulated by the noble metal nanoparticles further focus on taking the hollow porous polymer nanosphere composite material encapsulated by palladium metal nanoparticles as a heterogeneous catalyst, and solvent polarity-induced catalytic hydrogenation application with adjustable size selectivity is provided.
Owner:EAST CHINA NORMAL UNIV
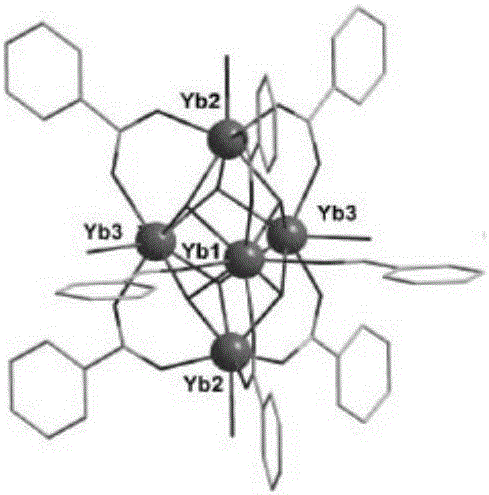
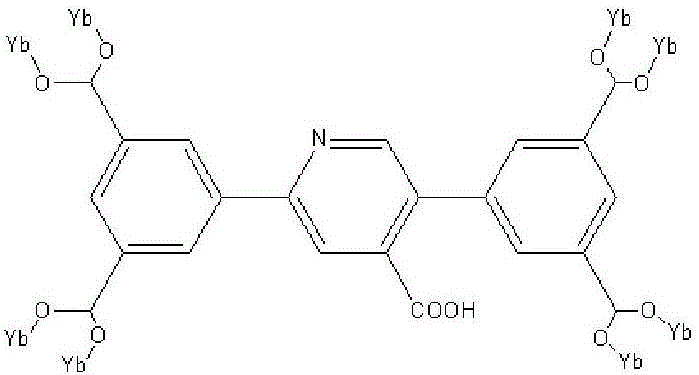
Metallic organic frame material based on YbIII pentanuclear molecular structure unit, preparation method and application thereof
InactiveCN106748994AImprove catalytic performanceGood size selective catalytic performanceOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCycloadditionRare earth
The invention relates to a metallic organic frame material based on a YbIII pentanuclear molecular structure unit, a preparation method and an application thereof. According to the technical scheme, the method comprises the following steps: adding Yb(NO3)3, ligand 5,5'-(4-carboxyl-pyridyl-2,5-bis-substituted)-1,3-phthalic acid, solvent N,N-dimethyl formamide and water into a high-temperature resisting spiral opening glass bottle; stirring for 30 minutes under normal temperature; sealing the container and then putting into an oven; keeping for 4 days under the temperature at 378K; slowly cooling to room temperature, thereby acquiring a colorless transparent block crystal; washing with N,N-dimethyl formamide, filtering and drying, thereby acquiring a target product. The metallic organic frame material based on the YbIII pentanuclear molecular structure unit prepared according to the invention is simple in preparation method and has an excellent size selective catalytic performance for the carbon dioxide cycloaddition reaction of the epoxide.
Owner:LIAONING UNIVERSITY
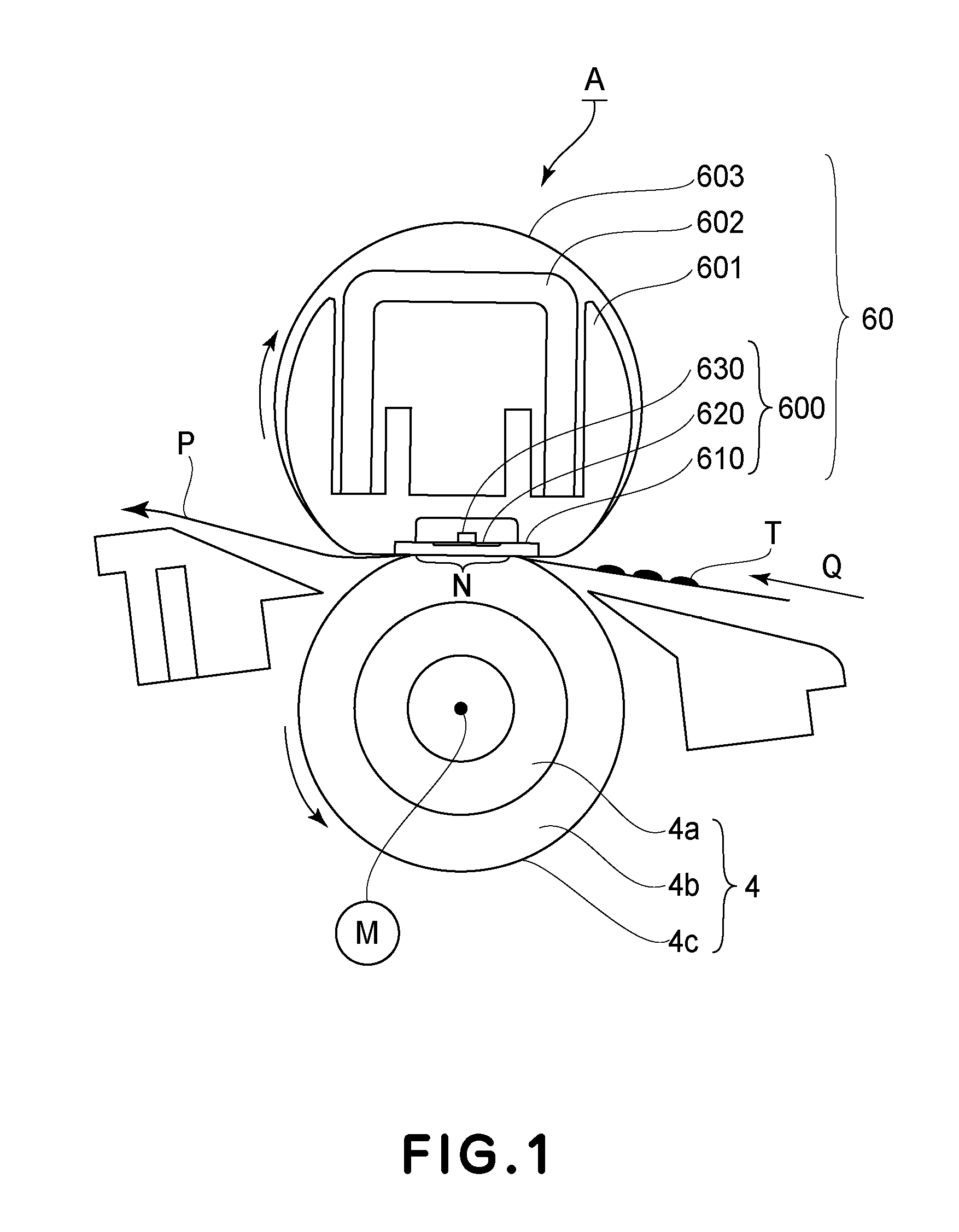
Fixing device
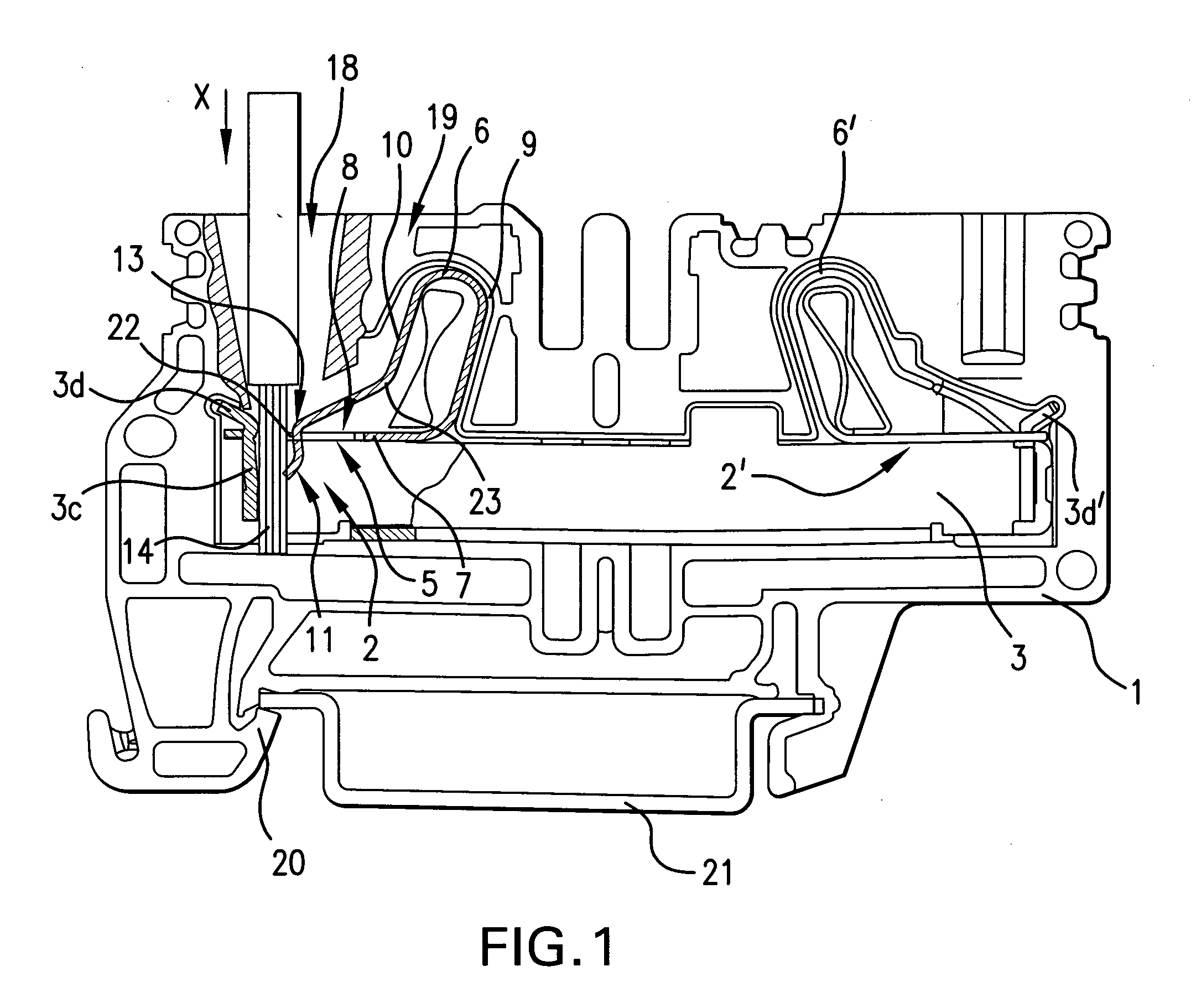
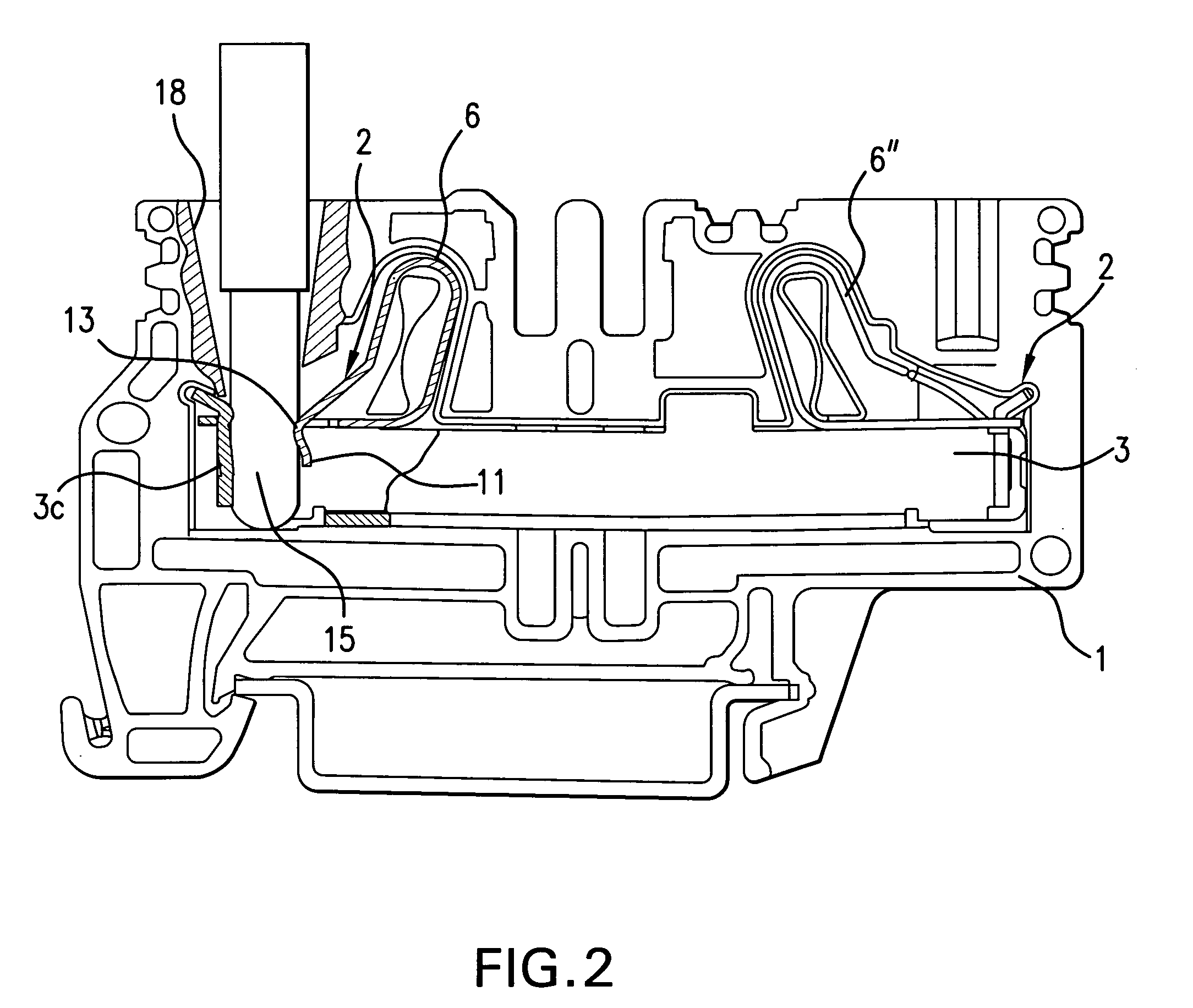
A fixing device includes a rotatable heating member and a rotatable pressing member which are configured to fix a toner image on a recording material in a nip therebetween; and a heating mechanism configured to selectively heat regions of the rotatable heating member depending on sizes of the recording material when the sizes are predetermined ones of all sizes usable in the fixing device. The rotatable pressing member includes a base layer and a porous elastic layer provided on the base layer and containing a needle-like filler. The elastic layer having a thermal conductivity, with respect to a longitudinal direction thereof, which is 6 times to 900 times a thermal conductivity with respect to a thickness direction thereof.
Owner:CANON KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com