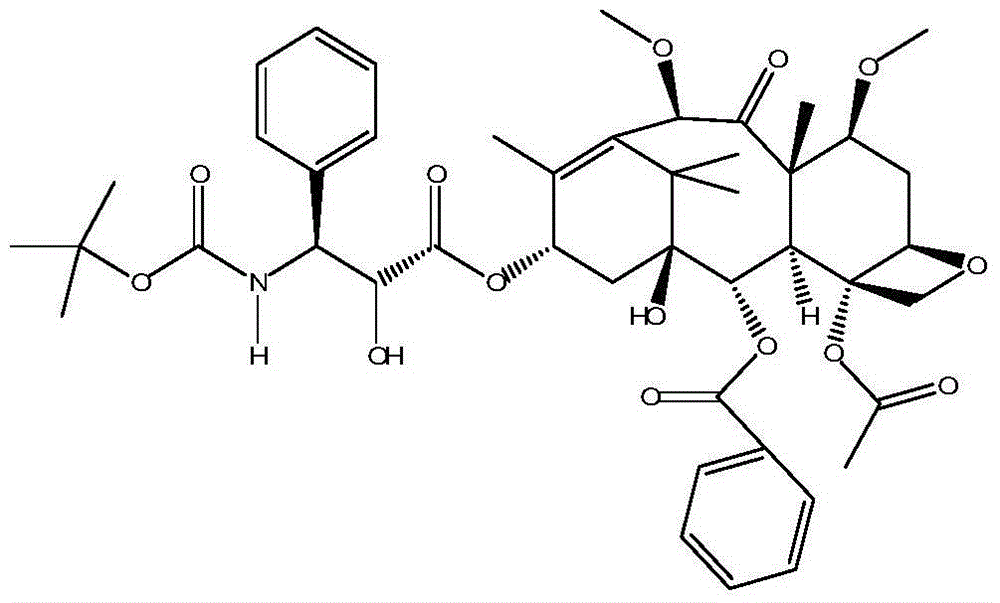
Preparation method of amorphous cabazitaxel
An amorphous technology of cabazitaxel, which is applied in the field of preparation of taxane compounds, can solve the problem that the residual amount of solvent is difficult to reach a high standard, and achieve the effect of good and stable physical and chemical state and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
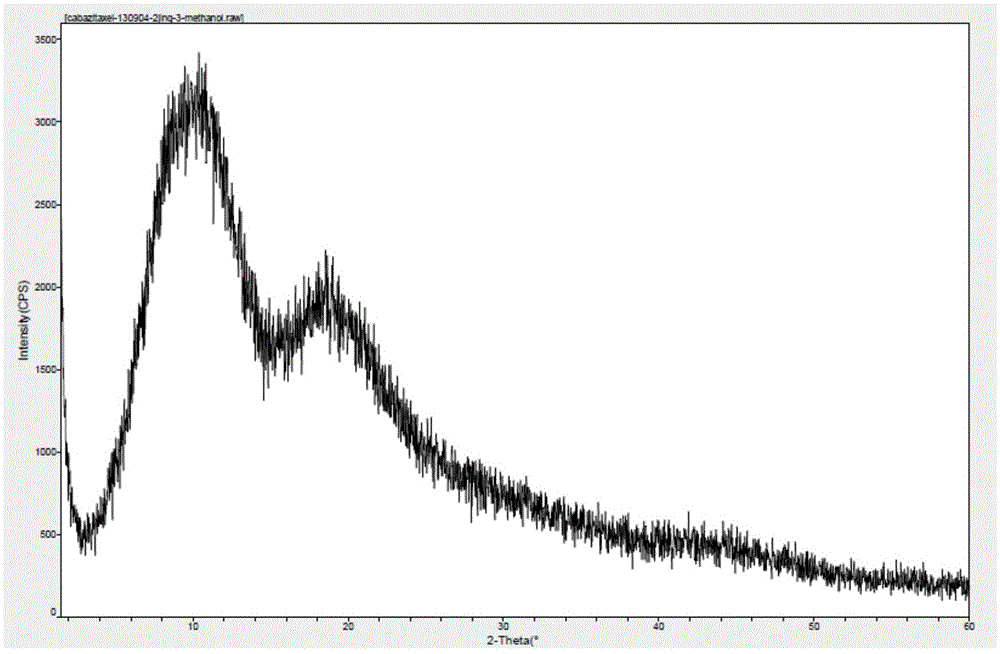
[0036] The preparation of embodiment 1 amorphous cabazitaxel
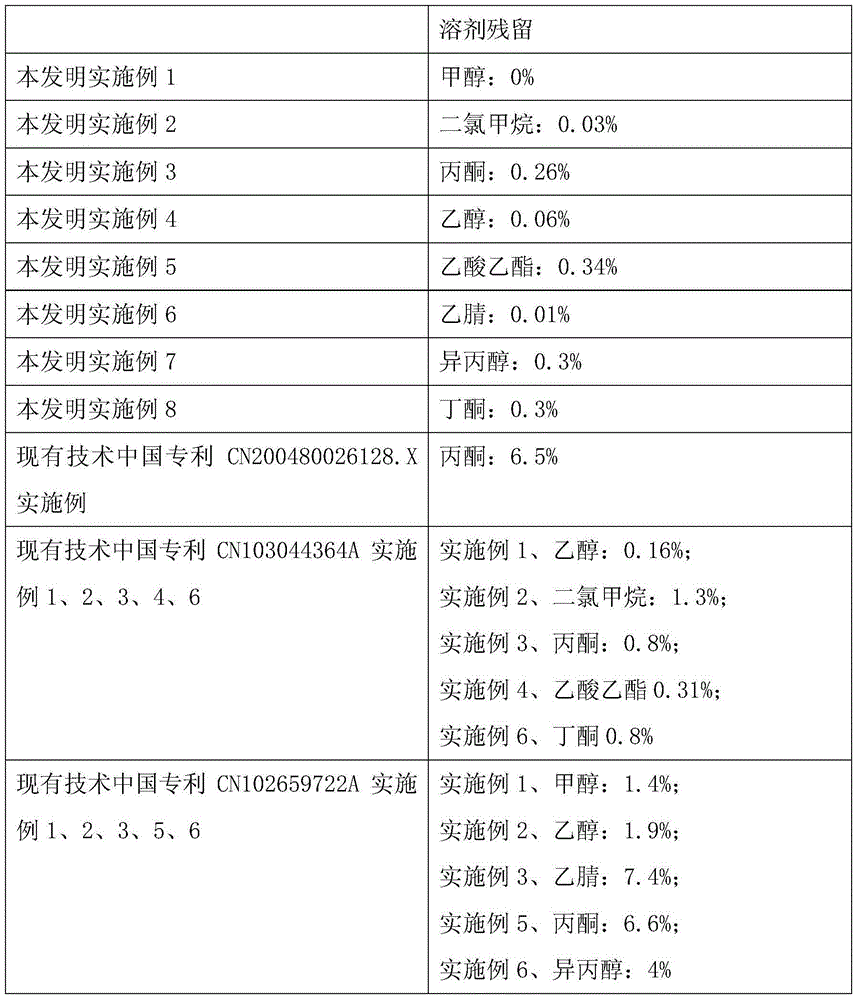
[0037] Dissolve 1 g of cabazitaxel in 10 ml of methanol, place it in a watch glass, volatilize under normal pressure at 30°C, and dry at elevated temperature at 40°C to obtain 0.9 g of amorphous cabazitaxel. Methanol solvent residue: 0% (limit 0.3%)
Embodiment 2
[0038] The preparation of embodiment 2 amorphous cabazitaxel
[0039] Dissolve 1 g of cabazitaxel in 10 ml of dichloromethane, put it in a watch glass, volatilize under normal pressure at 30 ° C, and dry at 40 ° C to obtain 0.9 g of amorphous cabazitaxel. Dichloromethane solvent residue: 0.03% (limit 0.06%)
Embodiment 3
[0040] The preparation of embodiment 3 amorphous cabazitaxel
[0041] Dissolve 1 g of cabazitaxel in 10 ml of acetone, put it in a watch glass, dry it under reduced pressure at 30°C, and dry at 40°C to obtain 0.9 g of amorphous cabazitaxel. Acetone solvent residue: 0.26% (limit 0.5%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com