Application of a Nanocomposite of Functionalized Polyamide-Amine Dendrimers
A technology of amine dendrimers and nanocomposites, applied in the field of targeted gene transfection with polymer nanocarriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Weigh NH 2 - PEG-COOH 11.58 mg, dissolved in 5 mL DMSO. While stirring, 6-MAL with a dry weight of 1.785 mg dissolved in 1 mL of DMSO solution was added dropwise, and reacted for 8 hours to obtain a MAL-PEG-COOH solution. Add 1mL RGD-SH DMSO solution (4mg / mL) dropwise to the above MAL-PEG-COOH solution, and react for 12h to obtain RGD-PEG-COOH solution. Weigh 4.0 mg of EDC and 2.4 mg of NHS, dissolve them in 1 mL of DMSO, add the EDC solution and NHS solution drop by drop to the RGD-PEG-COOH solution, and stir for 3 hours. Weigh the fifth generation polyamidoamine dendrimer (G5.NH 2 ) 15.06 mg, dissolved in 5 mL DMSO. Add the activated RGD-PEG-COOH solution dropwise to G5.NH 2 solution, magnetically stirred at room temperature, and reacted for 3d to obtain G5.NH 2 -(PEG-RGD) 10 . Weigh mPEG-COOH11.58mg, dissolved in 5mLDMSO. Weigh 2.0 mg EDC and 1.2 mg NHS, dissolve them in 1 mL DMSO respectively, add the EDC solution and NHS solution dropwise to the mPEG-COOH s...
Embodiment 2
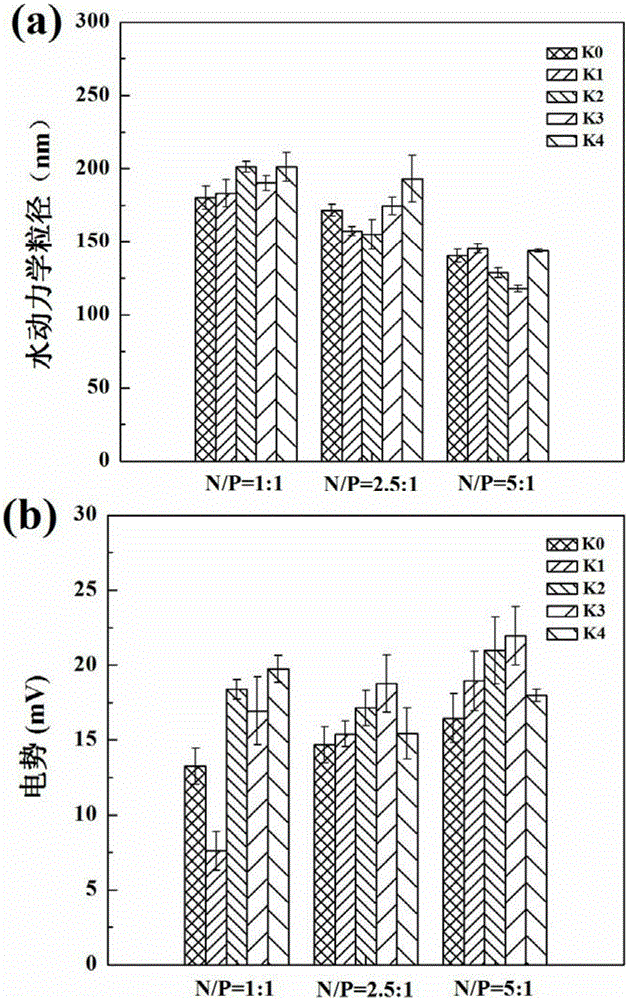
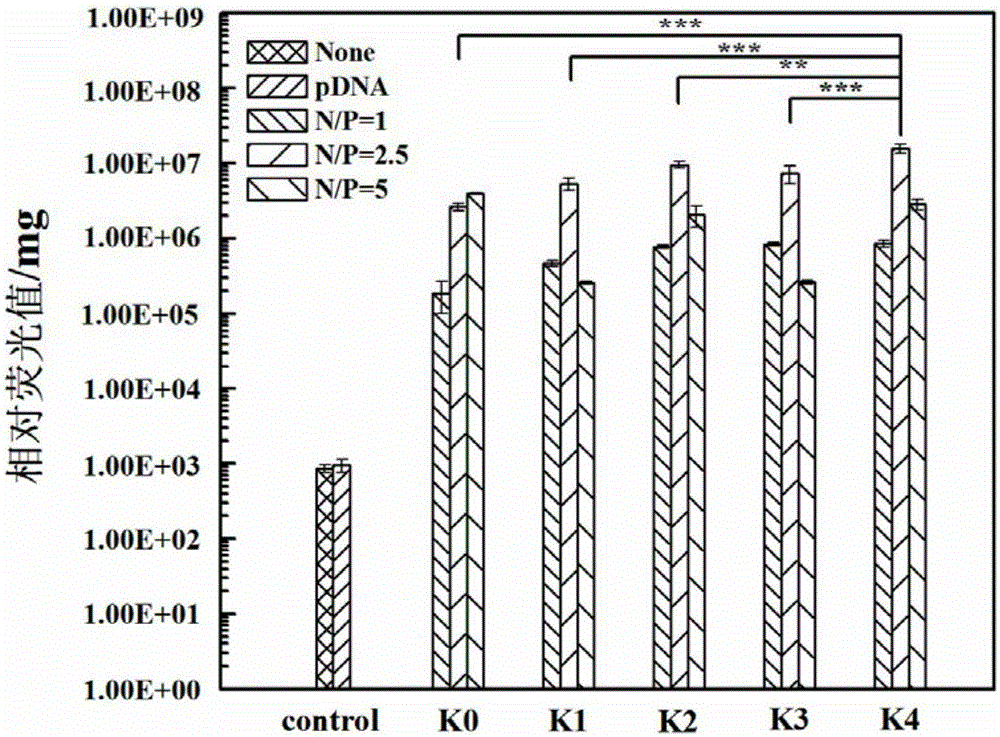
[0072] G5.NH 2 , Comparative Example 1, Comparative Example 2, the G5.NH prepared by the method of Comparative Example 3 and Example 1 2 -mPEG 20 , {(Au 0 ) 25 -G5.NH 2 -mPEG 20}, G5.NH 2 -(PEG-RGD) 10 -mPEG 10 , {(Au 0 ) 25 -G5.NH 2 -(PEG-RGD) 10 -mPEG 10}Under different N / P ratio conditions (1:1, 2.5:1, 5:1) respectively form vector / hBMP-2pDNA complexes with 5 μghBMP-2pDNA through electrostatic interaction, so that the final volume is fixed at 100 μL, and incubated at room temperature 20min, then add 1mL LPBS. Its hydrodynamic particle size and surface potential were characterized by Malvern laser particle size analyzer (Malvern, MK, 633nm laser), the results are as attached to the description figure 1 shown. The results showed that with the increase of N / P ratio, the hydrodynamic particle size of the composites decreased, almost all below 200nm; while the surface potential of most of the composites increased with the increase of N / P ratio, but Also around 20...
Embodiment 3
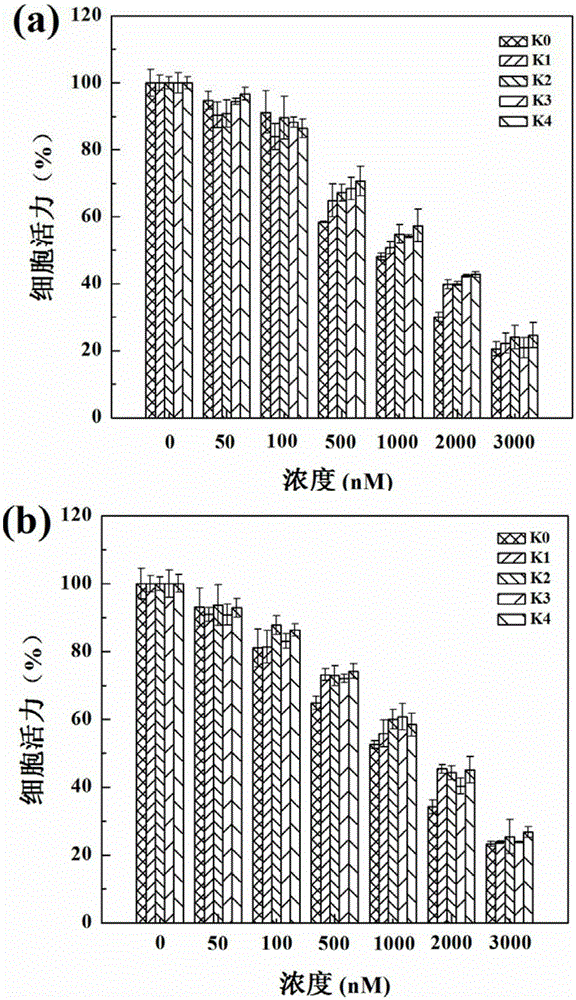
[0074] to have V 3 Bone marrow mesenchymal stem cells hMSCs expressing integrin as model cells to test G5.NH 2 , the cytotoxicity of the materials prepared in Comparative Example 1, Comparative Example 2, Comparative Example 3 and Example 1. Take 1.5×10 4 Density per well hMSCs were seeded in 96-well plates, cultured in 100 μL α-MEM medium supplemented with 100 U / mL penicillin, 100 U / mL streptomycin and 10% FBS, and cultured at 37 °C for 24 h at a concentration of 5% carbon dioxide. Then the medium was replaced with G5.NH containing G5. 2 , G5.NH 2 -mPEG 20 , {(Au 0 ) 25 -G5.NH 2 -mPEG 20}, G5.NH 2 -(PEG-RGD) 10 -mPEG 10 and {(Au 0 ) 25 -G5.NH 2 -(PEG-RGD) 10 -mPEG 10} cell culture medium and cells were co-cultured for 24 hours, then 20 μL of MTT (5.0 mg / mL) solution was added, and the culture was continued for 4 hours. Remove the culture medium in the wells, add 150 μL DMSO to each well, shake on the shaker for 30 minutes, and test the absorbance value with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com