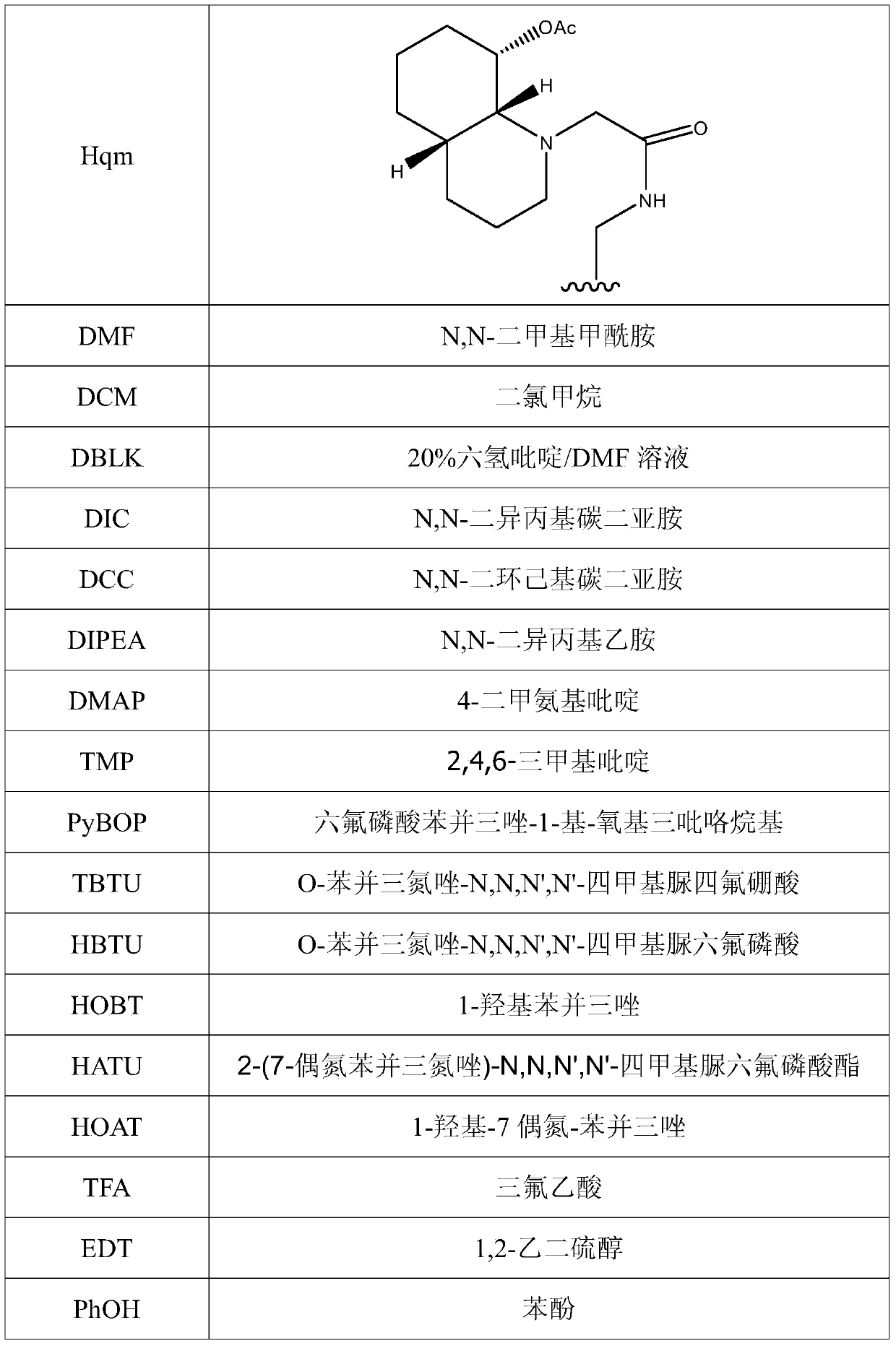
Method for preparing linaclotide
A technology of linaclotide and crude peptide, which is applied in the field of preparation of linaclotide, can solve the problems of inability to form three pairs of disulfide bonds with complete selectivity, unable to obtain target products, unsatisfactory results, etc., and achieves considerable economy. The effect of practical value, easy post-processing and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of the Fmoc-Tyr(tBu)-Wang Resin that the degree of substitution is 0.51mmol / g
[0037]Weigh 100 grams of Wang Resin with a substitution degree of 1.0 mmol / g in a solid-phase reaction column, add DMF, and swell with nitrogen bubbles for 60 minutes; weigh 45.9 grams (100 mmol) of Fmoc-Tyr(tBu)-OH, HOBt16.2 gram (120mmol), DMAP 1.2g (10mmol), dissolved in DMF, 20.3ml DIC (120mmol) was added in an ice-water bath at 0°C, activated for 5 minutes, added to the reaction column, after 2 hours of reaction, 70ml acetic anhydride and 60ml pyridine were added , mixed and sealed for 24 hours, washed with DCM three times, and the resin was dried after shrinking with methanol to obtain Fmoc-Tyr(tBu)-Wang Resin, and the detection substitution degree was 0.51mmol / g.
Embodiment 2
[0038] Embodiment 2: the preparation of the Fmoc-Tyr(tBu)-Wang Resin that the degree of substitution is 0.56mmol / g
[0039] Weigh 100 grams of Wang Resin with a substitution degree of 1.0 mmol / g in a solid-phase reaction column, add DMF, and swell with nitrogen bubbles for 60 minutes; weigh 45.9 grams (100 mmol) of Fmoc-Tyr(tBu)-OH, HOBt16.2 gram (120mmol), DMAP1.2g (10mmol), dissolved in DMF, 40.6ml DIC (240mmol) was added in ice-water bath at 0°C, activated for 5 minutes, added to the reaction column, after 2 hours of reaction, added 70ml acetic anhydride and 60ml pyridine , mixed and blocked for 24 hours, washed with DCM three times, dried the resin after shrinking with methanol to obtain Fmoc-Tyr(tBu)-Wang Resin, and the detection substitution degree was 0.56mmol / g.
Embodiment 3
[0040] Embodiment 3: the preparation of the Fmoc-Tyr(tBu)-Wang Resin that the degree of substitution is 0.63mmol / g
[0041] Weigh 100 grams of Wang Resin with a substitution degree of 1.0 mmol / g in a solid-phase reaction column, add DMF, and swell with nitrogen bubbles for 60 minutes; weigh 45.9 grams (100 mmol) of Fmoc-Tyr(tBu)-OH, HOBt16.2 gram (120mmol), HBTU38.0g (100mmol), DMAP1.2g (10mmol), dissolved in DMF, added 25.6ml DIPEA (120mmol) in ice-water bath at 0°C, activated for 5 minutes, added to the reaction column, reacted for 2 hours , add 70ml of acetic anhydride and 60ml of pyridine, mix and seal for 24h, wash with DCM three times, drain the resin after shrinking with methanol, and obtain Fmoc-Tyr(tBu)-Wang Resin, the detection substitution degree is 0.63mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com