Acylated derivatives of homoharringtonine, its preparation method and application
The technology of homoharringtonine and harringtonine acyl is applied in the fields of natural medicine and medicinal chemistry, and can solve the problems of synthesis and application of new homoharringtonine derivatives that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Embodiment 1: the synthesis of compound BS-HH-001
[0135]
[0136] In the formula, HHT: homoharringtonine; Ac 2 O: acetic anhydride; DIPEA: N,N-diisopropylethylamine; DMAP: 4-dimethylaminopyridine; DCM: dichloromethane; BS-HH-001: 2,6-diacetylhomoharringtonate base; BS-HH-002: 6-acetylhomoharringtonine.
[0137] Homoharringtonine HHT (125 mg, 0.23 mmol), diisopropylethylamine (444 mg, 3.44 mmol) and 4-dimethylaminopyridine (28 mg, 0.023 mmol) were dissolved in dichloromethane (2 mL), and Add acetic anhydride (351mg, 3.44mmol) to the mixed solution, react at 35°C for 24 hours, wash the reaction solution with water, and then wash with saturated sodium bicarbonate, dry, concentrate, and the obtained crude product is purified by preparative liquid chromatography , to obtain white solid compound BS-HH-001 (43.3 mg, 30%).
[0138] LC-MS: retention time: 1.11min (98.7%), m / z: 630.5[M+H] + . 1 HNMR (300MHz, CDCl 3 ): δ6.60(d, 2H), 5.80-5.93(m, 3H), 5.02(s, 1H), 3.76(d...
Embodiment 2
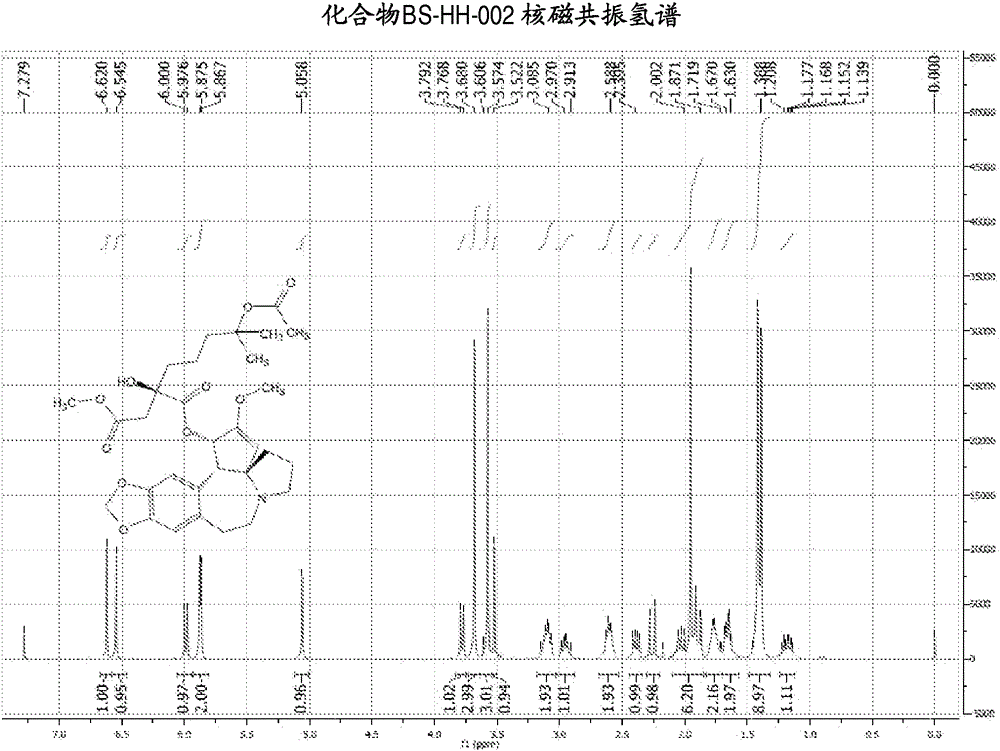
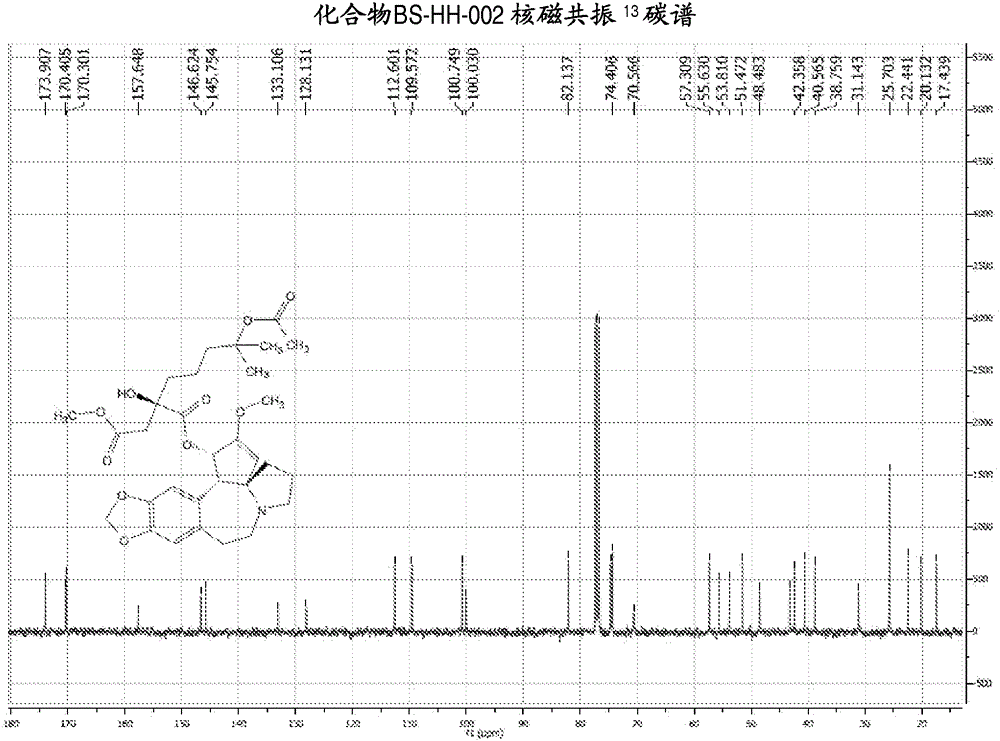
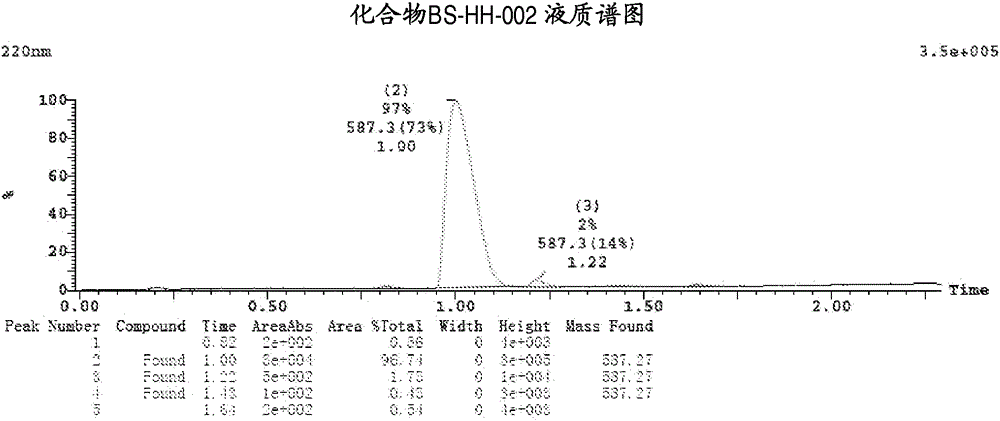
[0139] Embodiment 2: the synthesis of compound BS-HH-002
[0140]
[0141] In the formula, HHT: homoharringtonine; Ac 2 O: acetic anhydride;
[0142] to Ac 2 Homoharringtonine HHT (1g, 1.84mmol) was added to O (20mL), the reaction was heated to 80°C, and the reaction was stirred for 13 hours. After the reaction was completed, the reaction solution was concentrated to obtain a viscous crude product, which was solidified after adding diethyl ether. Ethyl acetate was added to the solidified crude product to dissolve it, washed with saturated potassium carbonate solution, concentrated, and the obtained crude product was purified by a silica gel column (EA:PE=1:2) to obtain a yellow oil, which was recrystallized with ether A white solid compound BS-HH-002 (654 mg, 61%) was obtained, while homoharringtonine HHT starting material (354 mg) was recovered. BS-HH-002: LC-MS: retention time: 1.00min (96.74%), m / z: 588.2[M+H] + . 1 HNMR (400MHz, CDCl 3 ): δ6.62(s, 1H), 6.55(s, 1H)...
Embodiment 3
[0144] Embodiment 3: the synthesis of compound BS-HH-077
[0145]
[0146] In the formula, HHT: homoharringtonine; Yi: cyclopentylcarboxylic acid; 4-ppy: 4-(1′-tetrahydropyrrole) pyridine; DCC: dicyclohexylcarbodiimide; DCM: dichloromethane.
[0147] Dissolve homoharringtonine (110 mg, 0.2 mmol), cyclopentylcarboxylic acid (46 mg, 0.4 mmol), 4-(1'-tetrahydropyrrole) pyridine (60 mg, 0.4 mmol) in dichloromethane (2 mL) , dicyclohexylcarbodiimide (83mg, 0.4mmol) was added to the solution, heated to reflux for three hours, and then filtered. The filtrate was concentrated, and the obtained crude product was purified by high performance liquid chromatography to obtain BS-HH-077 (35.9 mg, 28%) as a light yellow powdery solid.
[0148] LC-MS: retention time: 1.52min (95.63%), m / z: 642.6[M+H] + . 1 HNMR (300MHz, CDCl 3 ): δ6.63(s, 1H), 6.59(s, 1H), 5.99(d, J=9.0Hz, 1H), 5.90(m, 2H), 5.09(s, 1H), 3.80(m, 1H) , 3.73(s, 3H), 3.57(s, 3H), 3.49-3.54(m, 1H), 2.73-3.02(m, 2H), 2.42-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com